Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis
- PMID: 31019198
- PMCID: PMC6482305
- DOI: 10.1038/s41598-019-42751-4
Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis
Erratum in
-
Author Correction: Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis.Sci Rep. 2019 Nov 21;9(1):17535. doi: 10.1038/s41598-019-54253-4. Sci Rep. 2019. PMID: 31754179 Free PMC article.
Abstract
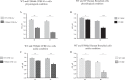
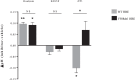
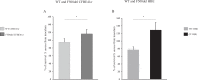
Cystic fibrosis (CF) is caused by defective Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) protein. Morbidity is mainly due to early airway infection. We hypothesized that S. aureus clearance during the first hours of infection was impaired in CF human Airway Surface Liquid (ASL) because of a lowered pH. The ASL pH of human bronchial epithelial cell lines and primary respiratory cells from healthy controls (WT) and patients with CF was measured with a pH microelectrode. The antimicrobial capacity of airway cells was studied after S. aureus apical infection by counting surviving bacteria. ASL was significantly more acidic in CF than in WT respiratory cells. This was consistent with a defect in bicarbonate secretion involving CFTR and SLC26A4 (pendrin) and a persistent proton secretion by ATP12A. ASL demonstrated a defect in S. aureus clearance which was improved by pH normalization. Pendrin inhibition in WT airways recapitulated the CF airway defect and increased S. aureus proliferation. ATP12A inhibition by ouabain decreased bacterial proliferation. Antimicrobial peptides LL-37 and hBD1 demonstrated a pH-dependent activity. Normalizing ASL pH might improve innate airway defense in newborns with CF during onset of S. aureus infection. Pendrin activation and ATP12A inhibition could represent novel therapeutic strategies to normalize pH in CF airways.
Conflict of interest statement
The authors declare no competing interests.
Figures


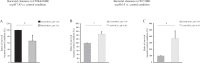

Similar articles
-
V-Type ATPase Mediates Airway Surface Liquid Acidification in Pig Small Airway Epithelial Cells.Am J Respir Cell Mol Biol. 2021 Aug;65(2):146-156. doi: 10.1165/rcmb.2020-0349OC. Am J Respir Cell Mol Biol. 2021. PMID: 33789071 Free PMC article.
-
Inflammatory cytokines TNF-α and IL-17 enhance the efficacy of cystic fibrosis transmembrane conductance regulator modulators.J Clin Invest. 2021 Aug 16;131(16):e150398. doi: 10.1172/JCI150398. J Clin Invest. 2021. PMID: 34166230 Free PMC article.
-
Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium.Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):16083-8. doi: 10.1073/pnas.2634339100. Epub 2003 Dec 10. Proc Natl Acad Sci U S A. 2003. PMID: 14668433 Free PMC article.
-
Airway Surface Liquid pH Regulation in Airway Epithelium Current Understandings and Gaps in Knowledge.Int J Mol Sci. 2021 Mar 25;22(7):3384. doi: 10.3390/ijms22073384. Int J Mol Sci. 2021. PMID: 33806154 Free PMC article. Review.
-
Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets.Thorax. 2016 Mar;71(3):284-7. doi: 10.1136/thoraxjnl-2015-207588. Epub 2015 Dec 30. Thorax. 2016. PMID: 26719229 Review.
Cited by
-
Alkalosis-induced hypoventilation in cystic fibrosis: The importance of efficient renal adaptation.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2116836119. doi: 10.1073/pnas.2116836119. Proc Natl Acad Sci U S A. 2022. PMID: 35173044 Free PMC article.
-
The Effect of CFTR Modulators on Airway Infection in Cystic Fibrosis.Int J Mol Sci. 2022 Mar 23;23(7):3513. doi: 10.3390/ijms23073513. Int J Mol Sci. 2022. PMID: 35408875 Free PMC article. Review.
-
Increased susceptibility of cystic fibrosis airway epithelial cells to ferroptosis.Biol Res. 2021 Dec 13;54(1):38. doi: 10.1186/s40659-021-00361-3. Biol Res. 2021. PMID: 34903297 Free PMC article.
-
Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic.BMC Pulm Med. 2021 May 20;21(1):173. doi: 10.1186/s12890-021-01528-0. BMC Pulm Med. 2021. PMID: 34016096 Free PMC article.
-
Epidemiological Profile of Hospitalized Patients with Cystic Fibrosis in Brazil Due to Severe Acute Respiratory Infection during the COVID-19 Pandemic and a Systematic Review of Worldwide COVID-19 in Those with Cystic Fibrosis.Healthcare (Basel). 2023 Jul 4;11(13):1936. doi: 10.3390/healthcare11131936. Healthcare (Basel). 2023. PMID: 37444770 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

