Glucagon reduces airway hyperreactivity, inflammation, and remodeling induced by ovalbumin
- PMID: 31019244
- PMCID: PMC6482309
- DOI: 10.1038/s41598-019-42981-6
Glucagon reduces airway hyperreactivity, inflammation, and remodeling induced by ovalbumin
Abstract
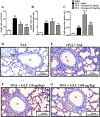
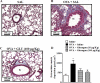
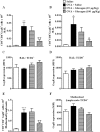
Glucagon has been shown to be beneficial as a treatment for bronchospasm in asthmatics. Here, we investigate if glucagon would prevent airway hyperreactivity (AHR), lung inflammation, and remodeling in a murine model of asthma. Glucagon (10 and 100 µg/Kg, i.n.) significantly prevented AHR and eosinophilia in BAL and peribronchiolar region induced by ovalbumin (OVA) challenge, while only the dose of 100 µg/Kg of glucagon inhibited subepithelial fibrosis and T lymphocytes accumulation in BAL and lung. The inhibitory action of glucagon occurred in parallel with reduction of OVA-induced generation of IL-4, IL-5, IL-13, TNF-α, eotaxin-1/CCL11, and eotaxin-2/CCL24 but not MDC/CCL22 and TARC/CCL17. The inhibitory effect of glucagon (100 µg/Kg, i.n.) on OVA-induced AHR and collagen deposition was reversed by pre-treatment with indomethacin (10 mg/Kg, i.p.). Glucagon increased intracellular cAMP levels and inhibits anti-CD3 plus anti-CD28-induced proliferation and production of IL-2, IL-4, IL-10, and TNF- α from TCD4+ cells in vitro. These findings suggest that glucagon reduces crucial features of asthma, including AHR, lung inflammation, and remodeling, in a mechanism probably associated with inhibition of eosinophils accumulation and TCD4+ cell proliferation and function. Glucagon should be further investigated as an option for asthma therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma.Int Immunopharmacol. 2011 Aug;11(8):899-906. doi: 10.1016/j.intimp.2011.02.001. Epub 2011 Feb 26. Int Immunopharmacol. 2011. PMID: 21354484
-
The presence of LPS in OVA inhalations affects airway inflammation and AHR but not remodeling in a rodent model of asthma.Am J Physiol Lung Cell Mol Physiol. 2012 Jul 1;303(1):L54-63. doi: 10.1152/ajplung.00208.2011. Epub 2012 Apr 20. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22523281
-
MFG-E8/integrin β3 signaling contributes to airway inflammation response and airway remodeling in an ovalbumin-induced murine model of asthma.J Cell Biochem. 2018 Nov;119(11):8887-8896. doi: 10.1002/jcb.27142. Epub 2018 Aug 4. J Cell Biochem. 2018. PMID: 30076648
-
4-1 BB stimulation inhibits allergen-specific immunoglobulin E production and airway hyper-reactivity but partially suppresses bronchial eosinophilic inflammation in a mouse asthma model.Clin Exp Allergy. 2006 Mar;36(3):377-85. doi: 10.1111/j.1365-2222.2006.02445.x. Clin Exp Allergy. 2006. PMID: 16499650
-
Allergic lung inflammation is mediated by soluble tumor necrosis factor (TNF) and attenuated by dominant-negative TNF biologics.Am J Respir Cell Mol Biol. 2011 Oct;45(4):731-9. doi: 10.1165/rcmb.2010-0512OC. Epub 2011 Feb 4. Am J Respir Cell Mol Biol. 2011. PMID: 21297077
Cited by
-
Resveratrol-Loaded Lipid-Core Nanocapsules Modulate Acute Lung Inflammation and Oxidative Imbalance Induced by LPS in Mice.Pharmaceutics. 2021 May 10;13(5):683. doi: 10.3390/pharmaceutics13050683. Pharmaceutics. 2021. PMID: 34068619 Free PMC article.
-
Physiological and Pathophysiological Roles of Metabolic Pathways for NET Formation and Other Neutrophil Functions.Front Immunol. 2022 Feb 9;13:826515. doi: 10.3389/fimmu.2022.826515. eCollection 2022. Front Immunol. 2022. PMID: 35251008 Free PMC article. Review.
-
Metabolic profiling in experimental guinea pig models of bacterial and allergic inflammation.Metabolomics. 2025 Mar 23;21(2):43. doi: 10.1007/s11306-025-02239-x. Metabolomics. 2025. PMID: 40123009 Free PMC article.
-
The Australasian dingo archetype: De novo chromosome-length genome assembly, DNA methylome, and cranial morphology.bioRxiv [Preprint]. 2023 Jan 27:2023.01.26.525801. doi: 10.1101/2023.01.26.525801. bioRxiv. 2023. Update in: Gigascience. 2023 Mar 20;12:giad018. doi: 10.1093/gigascience/giad018. PMID: 36747621 Free PMC article. Updated. Preprint.
-
Uncovering a Hub Signaling Pathway of Antimicrobial-Antifungal-Anticancer Peptides' Axis on Short Cationic Peptides via Network Pharmacology Study.Int J Mol Sci. 2022 Feb 12;23(4):2055. doi: 10.3390/ijms23042055. Int J Mol Sci. 2022. PMID: 35216171 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

