Intrathecal enzyme replacement for Hurler syndrome: biomarker association with neurocognitive outcomes
- PMID: 31019279
- PMCID: PMC6831510
- DOI: 10.1038/s41436-019-0522-1
Intrathecal enzyme replacement for Hurler syndrome: biomarker association with neurocognitive outcomes
Abstract
Purpose: Abnormalities in cerebrospinal fluid (CSF) have been reported in Hurler syndrome, a fatal neurodegenerative lysosomal disorder. While no biomarker has predicted neurocognitive response to treatment, one of these abnormalities, glycosaminoglycan nonreducing ends (NREs), holds promise to monitor therapeutic efficacy. A trial of intrathecal enzyme replacement therapy (ERT) added to standard treatment enabled tracking of CSF abnormalities, including NREs. We evaluated safety, biomarker response, and neurocognitive correlates of change.
Methods: In addition to intravenous ERT and hematopoietic cell transplantation, patients (N = 24) received intrathecal ERT at four peritransplant time points; CSF was evaluated at each point. Neurocognitive functioning was quantified at baseline, 1 year, and 2 years posttransplant. Changes in CSF biomarkers and neurocognitive function were evaluated for an association.
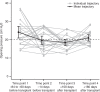
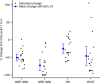
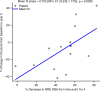
Results: Over treatment, there were significant decreases in CSF opening pressure, biomarkers of disease activity, and markers of inflammation. Percent decrease in NRE from pretreatment to final intrathecal dose posttransplant was positively associated with percent change in neurocognitive score from pretreatment to 2 years posttransplant.
Conclusion: Intrathecal ERT was safe and, in combination with standard treatment, was associated with reductions in CSF abnormalities. Critically, we report evidence of a link between a biomarker treatment response and neurocognitive outcome in Hurler syndrome.
Keywords: biomarkers; enzyme replacement therapy; intrathecal therapy; mucopolysaccharidosis; neurocognitive decline.
Conflict of interest statement
J.B.E. received honoraria, consulting fees, and/or research support from ArmaGen, Gene Spotlight, Inc., Sangamo, and Sanofi Genzyme, and has done contract work for Shapiro Neuropsychology Consulting LLC. K.E.K. is a consultant for Shire Plc; has received research support from Shire, Sanofi Genzyme, and Alexion Pharmaceuticals, Inc.; and has done previous contract work for Shapiro Neuropsychology Consulting. M.P. is a consultant for Moderna Tx, Inc. and BioMarin Pharmaceuticals, Inc. and has received travel support from BioMarin Pharmaceuticals, Inc. L.E.P. has received honoraria, consulting fees, and/or research support from Sanofi Genzyme, Shire, and BioMarin. P.I.D. receives research support from Biomarin and Genzyme. W.P.M. is an employee of Sangamo Therapeutics, Inc.. P.J.O. has received honoraria, consulting fees, and/or research support from Sanofi Genzyme, Bluebird Bio, and Horizon. T.C.L. has received speaker fees and research support from Sanofi Genzyme. The other authors declare no conflicts of interest.
Figures
Similar articles
-
Long-term outcomes of systemic therapies for Hurler syndrome: an international multicenter comparison.Genet Med. 2018 Nov;20(11):1423-1429. doi: 10.1038/gim.2018.29. Epub 2018 Mar 8. Genet Med. 2018. PMID: 29517765 Free PMC article.
-
Early enzyme replacement therapy enables a successful hematopoietic stem cell transplantation in mucopolysaccharidosis type IH: Divergent clinical outcomes in two Japanese siblings.Brain Dev. 2019 Jun;41(6):546-550. doi: 10.1016/j.braindev.2019.01.008. Epub 2019 Feb 10. Brain Dev. 2019. PMID: 30755342
-
Regression of ventriculomegaly following medical management of a patient with Hurler syndrome.J Neurosurg Pediatr. 2016 May;17(5):537-9. doi: 10.3171/2015.9.PEDS15477. Epub 2016 Jan 8. J Neurosurg Pediatr. 2016. PMID: 26745646
-
Intrathecal enzyme replacement therapy for mucopolysaccharidosis I: translating success in animal models to patients.Curr Pharm Biotechnol. 2011 Jun;12(6):946-55. doi: 10.2174/138920111795542642. Curr Pharm Biotechnol. 2011. PMID: 21506913 Review.
-
Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure.Orphanet J Rare Dis. 2011 Aug 10;6:55. doi: 10.1186/1750-1172-6-55. Orphanet J Rare Dis. 2011. PMID: 21831279 Free PMC article.
Cited by
-
Mucopolysaccharidosis Type I: Current Treatments, Limitations, and Prospects for Improvement.Biomolecules. 2021 Jan 29;11(2):189. doi: 10.3390/biom11020189. Biomolecules. 2021. PMID: 33572941 Free PMC article. Review.
-
Evolving therapies in neuronopathic LSDs: opportunities and challenges.Metab Brain Dis. 2022 Oct;37(7):2245-2256. doi: 10.1007/s11011-022-00939-0. Epub 2022 Apr 20. Metab Brain Dis. 2022. PMID: 35442005 Review.
-
Ionizable Lipid Nanoparticles for Therapeutic Base Editing of Congenital Brain Disease.ACS Nano. 2023 Jul 25;17(14):13594-13610. doi: 10.1021/acsnano.3c02268. Epub 2023 Jul 17. ACS Nano. 2023. PMID: 37458484 Free PMC article.
-
Pathogenic Roles of Heparan Sulfate and Its Use as a Biomarker in Mucopolysaccharidoses.Int J Mol Sci. 2022 Oct 3;23(19):11724. doi: 10.3390/ijms231911724. Int J Mol Sci. 2022. PMID: 36233030 Free PMC article. Review.
-
Hematopoietic stem cell transplant for Hurler syndrome: does using bone marrow or umbilical cord blood make a difference?Blood Adv. 2022 Dec 13;6(23):6023-6027. doi: 10.1182/bloodadvances.2022007212. Blood Adv. 2022. PMID: 35476057 Free PMC article. No abstract available.
References
-
- Neufeld E, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, editor. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 3421–3452.

