How macrophages deal with death
- PMID: 31019284
- PMCID: PMC6733267
- DOI: 10.1038/s41577-019-0167-y
How macrophages deal with death
Abstract
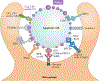
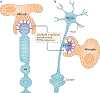
Tissue macrophages rapidly recognize and engulf apoptotic cells. These events require the display of so-called eat-me signals on the apoptotic cell surface, the most fundamental of which is phosphatidylserine (PtdSer). Externalization of this phospholipid is catalysed by scramblase enzymes, several of which are activated by caspase cleavage. PtdSer is detected both by macrophage receptors that bind to this phospholipid directly and by receptors that bind to a soluble bridging protein that is independently bound to PtdSer. Prominent among the latter receptors are the MER and AXL receptor tyrosine kinases. Eat-me signals also trigger macrophages to engulf virus-infected or metabolically traumatized, but still living, cells, and this 'murder by phagocytosis' may be a common phenomenon. Finally, the localized presentation of PtdSer and other eat-me signals on delimited cell surface domains may enable the phagocytic pruning of these 'locally dead' domains by macrophages, most notably by microglia of the central nervous system.
Conflict of interest statement
Competing interests
The author declares no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

