Population Pharmacokinetics of Vancomycin in the Pediatric Cardiac Surgical Population
- PMID: 31019403
- PMCID: PMC6478363
- DOI: 10.5863/1551-6776-24.2.107
Population Pharmacokinetics of Vancomycin in the Pediatric Cardiac Surgical Population
Abstract
Objective: Vancomycin is often used in the pediatric cardiac surgical population, but few pharmacokinetic data are available to guide dosing.
Methods: A retrospective, population pharmacokinetic study was performed for patients <19 years of age initiated on vancomycin after cardiac surgery in the cardiac intensive care unit from 2011-2016 in our institution. Patient data were summarized by using descriptive statistical methods, and population pharmacokinetic analysis was performed by using NONMEM. Simulation was performed to determine a dosing strategy that most frequently obtained an AUC0-24:MIC (minimum inhibitory concentration) ratio of >400.
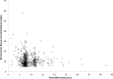
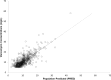
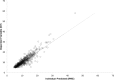
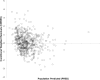
Results: A total of 261 patients (281 cardiac surgical procedures, cardiopulmonary bypass 82.3%) met inclusion criteria (60.1% male, median age 0.31 [IQR, 0.07-0.77] years). Vancomycin (14.5 ± 1.7 mg/kg/dose) was administered at median postoperative day 9 (IQR, 4-14), with a mean serum concentration of 11.5 ± 5.5 mg/L at 8.9 ± 3.8 hours after a dose. Population pharmacokinetic analysis demonstrated that a 1-compartment proportional error model with allometrically scaled weight best fit the data, with creatinine clearance and postmenstrual age as significant covariates. Simulation identified that a dosing regimen of 20 mg/kg/dose every 8 hours was most likely to achieve an AUC0-24:MIC ratio > 400 at a mean trough serum concentration of 12.9 ± 3.2 mg/L.
Conclusions: Vancomycin dosing in the postoperative pediatric cardiac surgical population should incorporate postmenstrual age and creatinine clearance. A vancomycin dose of 20 mg/kg every 8 hours is a reasonable empiric strategy.
Keywords: NONMEM; cardiac surgery; pediatrics; population pharmacokinetics; simulation; vancomycin.
Conflict of interest statement
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data and take responsibility for the integrity and accuracy of the data analysis.
Figures
Similar articles
-
Population pharmacokinetic analysis of vancomycin in pediatric continuous renal replacement therapy.Eur J Clin Pharmacol. 2019 Aug;75(8):1089-1097. doi: 10.1007/s00228-019-02664-7. Epub 2019 Apr 1. Eur J Clin Pharmacol. 2019. PMID: 30937470
-
Population Pharmacokinetics of Vancomycin in Pediatric Extracorporeal Membrane Oxygenation.Pediatr Crit Care Med. 2018 Oct;19(10):973-980. doi: 10.1097/PCC.0000000000001682. Pediatr Crit Care Med. 2018. PMID: 30063652
-
Phenobarbital population pharmacokinetics across the pediatric age spectrum.Epilepsia. 2018 Jul;59(7):1327-1333. doi: 10.1111/epi.14447. Epub 2018 Jun 13. Epilepsia. 2018. PMID: 29897629
-
The pharmacokinetic/pharmacodynamic rationale for administering vancomycin via continuous infusion.J Clin Pharm Ther. 2015 Jun;40(3):259-65. doi: 10.1111/jcpt.12270. Epub 2015 Apr 11. J Clin Pharm Ther. 2015. PMID: 25865426 Review.
-
AUC versus peak-trough dosing of vancomycin: applying new pharmacokinetic paradigms to an old drug.Ther Drug Monit. 2013 Aug;35(4):443-9. doi: 10.1097/FTD.0b013e31828b2a50. Ther Drug Monit. 2013. PMID: 23851909 Review.
Cited by
-
Pharmacokinetics of Commonly Used Medications in Children Receiving Continuous Renal Replacement Therapy: A Systematic Review of Current Literature.Clin Pharmacokinet. 2022 Feb;61(2):189-229. doi: 10.1007/s40262-021-01085-z. Epub 2021 Nov 30. Clin Pharmacokinet. 2022. PMID: 34846703 Free PMC article.
-
Dose Tailoring of Vancomycin Through Population Pharmacokinetic Modeling Among Surgical Patients in Pakistan.Front Pharmacol. 2021 Nov 11;12:721819. doi: 10.3389/fphar.2021.721819. eCollection 2021. Front Pharmacol. 2021. PMID: 34858169 Free PMC article.
-
Pharmacokinetics and Target Attainment of Antibiotics in Critically Ill Children: A Systematic Review of Current Literature.Clin Pharmacokinet. 2020 Feb;59(2):173-205. doi: 10.1007/s40262-019-00813-w. Clin Pharmacokinet. 2020. PMID: 31432468 Free PMC article.
-
Population Pharmacokinetics and Pharmacodynamics of Vancomycin in Pediatric Patients With Various Degrees of Renal Function.J Pediatr Pharmacol Ther. 2022;27(5):419-427. doi: 10.5863/1551-6776-27.5.419. Epub 2022 Jul 6. J Pediatr Pharmacol Ther. 2022. PMID: 35845555 Free PMC article.
-
External Validation of Model-Based Dosing Guidelines for Vancomycin, Gentamicin, and Tobramycin in Critically Ill Neonates and Children: A Pragmatic Two-Center Study.Paediatr Drugs. 2020 Aug;22(4):433-444. doi: 10.1007/s40272-020-00400-8. Paediatr Drugs. 2020. PMID: 32507958 Free PMC article.
References
-
- Abdel Hadi O, Al Omar S, Nazer LH et al. Vancomycin pharmacokinetics and predicted dosage requirements in pediatric cancer patients. J Oncol Pharm Pract. 2016;22(3):448–453. - PubMed
-
- Asbury WH, Darsey EH, Rose WB et al. Vancomycin pharmacokinetics in neonates and infants: a retrospective evaluation. Ann Pharmacother. 1993;27(4):490–496. - PubMed
-
- Guilhaumou R, Marsot A, Dupouey J et al. Pediatric patients with solid or hematological tumor disease: vancomycin population pharmacokinetics and dosage optimization. Ther Drug Monit. 2016;38(5):559–566. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
