Population Pharmacokinetics of Vancomycin in the Pediatric Cardiac Surgical Population
- PMID: 31019403
- PMCID: PMC6478363
- DOI: 10.5863/1551-6776-24.2.107
Population Pharmacokinetics of Vancomycin in the Pediatric Cardiac Surgical Population
Abstract
Objective: Vancomycin is often used in the pediatric cardiac surgical population, but few pharmacokinetic data are available to guide dosing.
Methods: A retrospective, population pharmacokinetic study was performed for patients <19 years of age initiated on vancomycin after cardiac surgery in the cardiac intensive care unit from 2011-2016 in our institution. Patient data were summarized by using descriptive statistical methods, and population pharmacokinetic analysis was performed by using NONMEM. Simulation was performed to determine a dosing strategy that most frequently obtained an AUC0-24:MIC (minimum inhibitory concentration) ratio of >400.
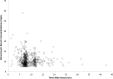
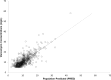
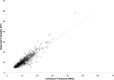
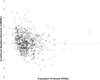
Results: A total of 261 patients (281 cardiac surgical procedures, cardiopulmonary bypass 82.3%) met inclusion criteria (60.1% male, median age 0.31 [IQR, 0.07-0.77] years). Vancomycin (14.5 ± 1.7 mg/kg/dose) was administered at median postoperative day 9 (IQR, 4-14), with a mean serum concentration of 11.5 ± 5.5 mg/L at 8.9 ± 3.8 hours after a dose. Population pharmacokinetic analysis demonstrated that a 1-compartment proportional error model with allometrically scaled weight best fit the data, with creatinine clearance and postmenstrual age as significant covariates. Simulation identified that a dosing regimen of 20 mg/kg/dose every 8 hours was most likely to achieve an AUC0-24:MIC ratio > 400 at a mean trough serum concentration of 12.9 ± 3.2 mg/L.
Conclusions: Vancomycin dosing in the postoperative pediatric cardiac surgical population should incorporate postmenstrual age and creatinine clearance. A vancomycin dose of 20 mg/kg every 8 hours is a reasonable empiric strategy.
Keywords: NONMEM; cardiac surgery; pediatrics; population pharmacokinetics; simulation; vancomycin.
Conflict of interest statement
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data and take responsibility for the integrity and accuracy of the data analysis.
Figures
References
-
- Abdel Hadi O, Al Omar S, Nazer LH et al. Vancomycin pharmacokinetics and predicted dosage requirements in pediatric cancer patients. J Oncol Pharm Pract. 2016;22(3):448–453. - PubMed
-
- Asbury WH, Darsey EH, Rose WB et al. Vancomycin pharmacokinetics in neonates and infants: a retrospective evaluation. Ann Pharmacother. 1993;27(4):490–496. - PubMed
-
- Guilhaumou R, Marsot A, Dupouey J et al. Pediatric patients with solid or hematological tumor disease: vancomycin population pharmacokinetics and dosage optimization. Ther Drug Monit. 2016;38(5):559–566. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
