Health Economics and Outcomes of Surfactant Treatments for Respiratory Distress Syndrome Among Preterm Infants in US Level III/IV Neonatal Intensive Care Units
- PMID: 31019404
- PMCID: PMC6478364
- DOI: 10.5863/1551-6776-24.2.117
Health Economics and Outcomes of Surfactant Treatments for Respiratory Distress Syndrome Among Preterm Infants in US Level III/IV Neonatal Intensive Care Units
Abstract
Objective: To compare length of stay (LOS), costs, mechanical ventilation (MV), and mortality in preterm infants treated in the Neonatal Intensive Care Unit (NICU) with beractant (BE), calfactant (CA), and poractant alfa (PA) for Respiratory Distress Syndrome (RDS).
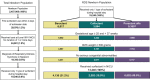
Methods: This study evaluated preterm infants born between 2010 and 2013 with RDS diagnosis, gestational age of 25 to 36 weeks, birthweight of ≥500 g, and age of ≤2 days on first surfactant administration. Multivariable regression was used to evaluate all NICU outcomes.
Results: Of 13,240 infants meeting the study criteria, 4136 (31.2%) received BE, 2502 (18.9%) received CA, and 6602 (49.9%) received PA. Adjusted analyses estimated similar mean LOS (BE 26.7 days, CA 27.8 days, and PA 26.2 days) and hospital costs (BE: $50,929; CA: $50,785; and PA: $50,212). Compared to PA, BE and CA were associated with greater odds of MV use on day 3 (OR = 1.56 and 1.60, respectively) and day 7 (OR = 1.39 and 1.28, respectively; all p < 0.05). Adjusted NICU mortality was significantly higher only with CA vs PA (OR = 1.51; p = 0.015).
Conclusion: Adjusted NICU LOS and costs were similar among BE, CA, and PA. Infants receiving PA were less likely to be on MV at 3 and 7 days, and PA treatment was associated with lower odds of NICU mortality when compared to CA.
Keywords: beractant; calfactant; costs; length of stay; mechanical ventilation; mortality; poractant alfa; respiratory distress syndrome; surfactant.
Conflict of interest statement
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. At the time of the study, MRK and FRE were employees of Premier Research Services, which contracted with Chiesi USA, the study sponsor to conduct the study; DF was an employee of Chiesi USA. The authors had full access to all the data and take responsibility for the integrity and accuracy of the data analysis.
Figures


References
-
- Sardesai S, Biniwale M, Wertheimer F et al. Evolution of surfactant therapy for respiratory distress syndrome: past, present, and future. Pediatr Res. 2017;81(1–2):240–248. - PubMed
-
- Respiratory distress syndrome. http://emedicine.medscape.com/article/976034 Accessed February 19, 2019.
-
- Martin R. Prevention and treatment of respiratory distress syndrome in preterm infants. In: Post T, editor. UpToDate. Waltham, MA: UpToDate; Sep 13, 2017. https://www.uptodate.com/contents/prevention-and-treatment-of-respirator... Accessed February 19, 2019.
-
- Preterm Birth: Causes, Consequences, and Prevention. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US); 2007. B, Prematurity at Birth: Determinants, Consequences, and Geographic Variation. https://www.ncbi.nlm.nih.gov/books/NBK11386/ Accessed October 21, 2017.
-
- Barfield WD. Levels of neonatal care. Pediatrics. 2012;130(3):587–597. - PubMed
LinkOut - more resources
Full Text Sources
