Graft-to-Recipient Weight Ratio Associated With Tacrolimus Metabolism Following Pediatric Living Donor Liver Transplantations
- PMID: 31019407
- PMCID: PMC6478353
- DOI: 10.5863/1551-6776-24.2.138
Graft-to-Recipient Weight Ratio Associated With Tacrolimus Metabolism Following Pediatric Living Donor Liver Transplantations
Abstract
Objectives: Tacrolimus (TAC) is an important immunosuppressant in liver transplantation. Since TAC is mainly metabolized by the liver enzymes CYP3A4 and 5, liver function is crucial for its pharmacokinetics (PK). Liver function is dynamic after liver transplantation; hence the PK of TAC metabolism after pediatric liver transplantation is not well understood. We aimed to investigate the time-dependent changes in TAC metabolism and to find factors influencing TAC PK after pediatric liver transplantation.
Methods: We retrospectively reviewed the characteristics of the donors and recipients in pediatric living donor liver transplantation and used the TAC concentration-dose (CD) ratio as a surrogate marker of TAC metabolism.
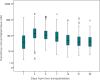
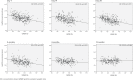
Results: Included were 326 patients with a median age of 13 months. After the liver transplantation, the CD ratio gradually decreased, then plateaued around day 21 to 28. A linear regression analysis demonstrated that a lower graft-to-recipient weight ratio (GRWR) and higher prothrombin time-international normalized ratio (PT-INR) were independently associated with a higher CD ratio in the early period after liver transplantation. However, association between GRWR and TAC CD ratio disappeared around 6 to 12 months after a liver transplantation possibly owing to graft regeneration.
Conclusions: Tacrolimus metabolism improved within the first month after liver transplantation, and the small graft size was associated with lower TAC metabolism in the early period after pediatric living donor liver transplantation.
Keywords: children; concentration-dose ratio; immunosuppressant; liver function; tacrolimus; time-dependent change.
Conflict of interest statement
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data and take responsibility for the integrity and accuracy of the data analysis. This study was supported by NCCHD grants 27-1 and 27-6 awarded to MK and IM, respectively.
Figures


Similar articles
-
Donor and recipient P450 gene polymorphisms influence individual pharmacological effects of tacrolimus in Chinese liver transplantation patients.Int Immunopharmacol. 2018 Apr;57:18-24. doi: 10.1016/j.intimp.2018.02.005. Epub 2018 Feb 15. Int Immunopharmacol. 2018. PMID: 29454235
-
Comparative clinical trial of the variability factors of the exposure indices used for the drug monitoring of two tacrolimus formulations in kidney transplant recipients.Pharmacol Res. 2018 Mar;129:84-94. doi: 10.1016/j.phrs.2017.12.005. Epub 2017 Dec 8. Pharmacol Res. 2018. PMID: 29229354 Clinical Trial.
-
Impact of CYP3A4*22 allele on tacrolimus pharmacokinetics in early period after renal transplantation: toward updated genotype-based dosage guidelines.Ther Drug Monit. 2013 Oct;35(5):608-16. doi: 10.1097/FTD.0b013e318296045b. Ther Drug Monit. 2013. PMID: 24052064
-
Conversion of twice-daily to once-daily tacrolimus is safe in stable adult living donor liver transplant recipients.Hepatobiliary Pancreat Dis Int. 2015 Aug;14(4):374-9. doi: 10.1016/s1499-3872(15)60378-2. Hepatobiliary Pancreat Dis Int. 2015. PMID: 26256081
-
Once-daily prolonged release tacrolimus in liver transplantation: Experts' literature review and recommendations.Liver Transpl. 2015 Oct;21(10):1312-21. doi: 10.1002/lt.24228. Liver Transpl. 2015. PMID: 26264233 Review.
Cited by
-
Population Pharmacokinetics and Dosing Optimization of Vancomycin in Pediatric Liver Transplant Recipients.Microbiol Spectr. 2021 Oct 31;9(2):e0046021. doi: 10.1128/Spectrum.00460-21. Epub 2021 Oct 6. Microbiol Spectr. 2021. PMID: 34612690 Free PMC article.
-
Population pharmacokinetics of everolimus in adult liver transplant patients: Comparison to tacrolimus disposition and extrapolation to pediatrics.Clin Transl Sci. 2022 Nov;15(11):2652-2662. doi: 10.1111/cts.13389. Epub 2022 Sep 14. Clin Transl Sci. 2022. PMID: 36004935 Free PMC article.
-
Tacrolimus Trough Concentrations are Not Impacted by Epstein-Barr Virus Serology and Viral Load in Pediatric Liver Transplant Recipients.Eur J Drug Metab Pharmacokinet. 2025 Jun 17. doi: 10.1007/s13318-025-00954-3. Online ahead of print. Eur J Drug Metab Pharmacokinet. 2025. PMID: 40528069
-
GRWR Correlates with the Metabolism of Tacrolimus after Pediatric Living Donor Liver Transplantation According to Donor CYP3A5 Polymorphism.Biomed Res Int. 2022 Oct 30;2022:7647754. doi: 10.1155/2022/7647754. eCollection 2022. Biomed Res Int. 2022. PMID: 36349313 Free PMC article.
-
Early impact of donor CYP3A5 genotype and Graft-to-Recipient Weight Ratio on tacrolimus pharmacokinetics in pediatric liver transplant patients.Sci Rep. 2021 Jan 11;11(1):443. doi: 10.1038/s41598-020-79574-7. Sci Rep. 2021. PMID: 33432012 Free PMC article.
References
-
- Kelly DA, Bucuvalas JC, Alonso EM et al. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19(8):798–825. - PubMed
-
- Kamdem LK, Streit F, Zanger UM et al. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem. 2005;51(8):1374–1381. - PubMed
-
- Wallemacq PE, Verbeeck RK. Comparative clinical pharmacokinetics of tacrolimus in paediatric and adult patients. Clin Pharmacokinet. 2001;40(4):283–295. - PubMed
-
- Sattler M, Guengerich FP, Yun CH et al. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20(5):753–761. - PubMed
LinkOut - more resources
Full Text Sources
