Evidence of Aquaporin 4 Regulation by Thyroid Hormone During Mouse Brain Development and in Cultured Human Glioblastoma Multiforme Cells
- PMID: 31019448
- PMCID: PMC6458270
- DOI: 10.3389/fnins.2019.00317
Evidence of Aquaporin 4 Regulation by Thyroid Hormone During Mouse Brain Development and in Cultured Human Glioblastoma Multiforme Cells
Abstract
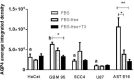
Accumulating evidence indicates that thyroid function and the thyroid hormones L-thyroxine (T4) and L-triiodothyronine (T3) are important factors contributing to the improvement of various pathologies of the central nervous system, including stroke, and various types of cancer, including glioblastoma multiforme (GBM). Low levels of T3 are correlated with the poorest outcome of post-stroke brain function, as well as an increased migration and proliferation of GBM tumor cells. Thyroid hormones are known to stimulate maturation and brain development. Aquaporin 4 (AQP4) is a key factor mediating the cell swelling and edema that occurs during ischemic stroke, and plays a potential role in the migration and proliferation of GBM tumor cells. In this study, as a possible therapeutic target for GBM, we investigated the potential role of T3 in the expression of AQP4 during different stages of mouse brain development. Pregnant mice at gestational day 18, or young animals at postnatal days 27 and 57, received injection of T3 (1 μg/g) or NaOH (0.02 N vehicle). The brains of mice sacrificed on postnatal days 0, 30, and 60 were perfused with 4% paraformaldehyde and sections were prepared for immunohistochemistry of AQP4. AQP4 immunofluorescence was measured in the mouse brains and human GBM cell lines. We found that distribution of AQP4 was localized in astrocytes of the periventricular, subpial, and cerebral parenchyma. Newborn mice treated with T3 showed a significant decrease in AQP4 immunoreactivity followed by an increased expression at P30 and a subsequent stabilization of aquaporin levels in adulthood. All GBM cell lines examined exhibited significantly lower AQP4 expression than cultured astrocytes. T3 treatment significantly downregulated AQP4 in GBM-95 cells but did not influence the rate of GBM cell migration measured 24 h after treatment initiation. Collectively, our results showed that AQP4 expression is developmentally regulated by T3 in astrocytes of the cerebral cortex of newborn and young mice, and is discretely downregulated in GBM cells. These findings indicate that higher concentrations of T3 thyroid hormone would be more suitable for reducing AQP4 in GBM tumorigenic cells, thereby resulting in better outcomes regarding the reduction of brain tumor cell migration and proliferation.
Keywords: GBM; aquaporin 4; brain development; brain tumor; thyroid hormone.
Figures





References
-
- Bergh J. J., Lin H. Y., Lansing L., Mohamed S. N., Davis F. B., Mousa S., et al. (2005). Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146 2864–2871. 10.1210/en.2005-0102 - DOI - PubMed
LinkOut - more resources
Full Text Sources

