Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults
- PMID: 31019473
- PMCID: PMC6459027
- DOI: 10.3389/fpsyt.2019.00207
Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults
Abstract
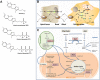
Thiamine (vitamin B1) is an essential nutrient that serves as a cofactor for a number of enzymes, mostly with mitochondrial localization. Some thiamine-dependent enzymes are involved in energy metabolism and biosynthesis of nucleic acids whereas others are part of the antioxidant machinery. The brain is highly vulnerable to thiamine deficiency due to its heavy reliance on mitochondrial ATP production. This is more evident during rapid growth (i.e., perinatal periods and children) in which thiamine deficiency is commonly associated with either malnutrition or genetic defects. Thiamine deficiency contributes to a number of conditions spanning from mild neurological and psychiatric symptoms (confusion, reduced memory, and sleep disturbances) to severe encephalopathy, ataxia, congestive heart failure, muscle atrophy, and even death. This review discusses the current knowledge on thiamine deficiency and associated morbidity of neurological and psychiatric disorders, with special emphasis on the pediatric population, as well as the putative beneficial effect of thiamine supplementation in autism spectrum disorder (ASD) and other neurological conditions.
Keywords: Krebs cycle; autism spectrum disorders; brain; depressive disorders; encephalomyopathies; pentose phosphate pathway; thiamine transporter.
Figures
References
-
- Martel JL, Franklin DS. Vitamin B1 (thiamine). In: StatPearls. Treasure Island (FL), (2018).
-
- Said HM, Nexo E. Chapter 64—Mechanisms and regulation of intestinal absorption of water-soluble vitamins: cellular and molecular aspects. In: Johnson LR, Ghishan FK, Kaunitz JD, Merchant JL, Said HM, Wood JD, editors. Physiology of the gastrointestinal tract, 5th ed Boston: Academic Press; (2012). p. 1711–56. 10.1016/B978-0-12-382026-6.00064-6 - DOI

