Necroptotic Cell Death Promotes Adaptive Immunity Against Colonizing Pneumococci
- PMID: 31019504
- PMCID: PMC6459137
- DOI: 10.3389/fimmu.2019.00615
Necroptotic Cell Death Promotes Adaptive Immunity Against Colonizing Pneumococci
Abstract
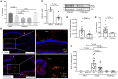
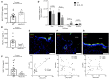
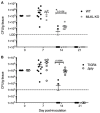
Pore-forming toxin (PFT) induced necroptosis exacerbates pulmonary injury during bacterial pneumonia. However, its role during asymptomatic nasopharyngeal colonization and toward the development of protective immunity was unknown. Using a mouse model of Streptococcus pneumoniae (Spn) asymptomatic colonization, we determined that nasopharyngeal epithelial cells (nEC) died of pneumolysin (Ply)-dependent necroptosis. Mice deficient in MLKL, the necroptosis effector, or challenged with Ply-deficient Spn showed less nEC sloughing, increased neutrophil infiltration, and altered IL-1α, IL-33, CXCL2, IL-17, and IL-6 levels in nasal lavage fluid (NALF). Activated MLKL correlated with increased presence of CD11c+ antigen presenting cells in Spn-associated submucosa. Colonized MLKL KO mice and wildtype mice colonized with Ply-deficient Spn produced less antibody against the bacterial surface protein PspA, were delayed in bacterial clearance, and were more susceptible to a lethal secondary Spn challenge. We conclude that PFT-induced necroptosis is instrumental in the natural development of protective immunity against opportunistic PFT-producing bacterial pathogens.
Keywords: Streptococcus pneumoniae; cell death; colonization; innate and adaptive immune response; necroptosis; necrosis; pneumolysin (PLY); pore-forming toxin (PFT).
Figures



Similar articles
-
The Yin and Yang of Pneumolysin During Pneumococcal Infection.Front Immunol. 2022 Apr 22;13:878244. doi: 10.3389/fimmu.2022.878244. eCollection 2022. Front Immunol. 2022. PMID: 35529870 Free PMC article. Review.
-
Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4.PLoS Pathog. 2010 Nov 11;6(11):e1001191. doi: 10.1371/journal.ppat.1001191. PLoS Pathog. 2010. PMID: 21085613 Free PMC article.
-
Microvesicles released from pneumolysin-stimulated lung epithelial cells carry mitochondrial cargo and suppress neutrophil oxidative burst.Sci Rep. 2021 May 5;11(1):9529. doi: 10.1038/s41598-021-88897-y. Sci Rep. 2021. PMID: 33953279 Free PMC article.
-
Otitis-prone Children Have Immunologic Deficiencies in Naturally Acquired Nasopharyngeal Mucosal Antibody Response after Streptococcus pneumoniae Colonization.Pediatr Infect Dis J. 2016 Jan;35(1):54-60. doi: 10.1097/INF.0000000000000949. Pediatr Infect Dis J. 2016. PMID: 26448450
-
The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae.Vaccine. 2000 Dec 8;19 Suppl 1:S87-95. doi: 10.1016/s0264-410x(00)00285-1. Vaccine. 2000. PMID: 11163470 Review.
Cited by
-
Mechanism of Bile Acid-Induced Programmed Cell Death and Drug Discovery against Cancer: A Review.Int J Mol Sci. 2022 Jun 28;23(13):7184. doi: 10.3390/ijms23137184. Int J Mol Sci. 2022. PMID: 35806184 Free PMC article. Review.
-
Necroptosis in bacterial infections.Front Immunol. 2024 Jun 12;15:1394857. doi: 10.3389/fimmu.2024.1394857. eCollection 2024. Front Immunol. 2024. PMID: 38933265 Free PMC article. Review.
-
Pneumolysin: Pathogenesis and Therapeutic Target.Front Microbiol. 2020 Jul 2;11:1543. doi: 10.3389/fmicb.2020.01543. eCollection 2020. Front Microbiol. 2020. PMID: 32714314 Free PMC article. Review.
-
Type I Interferon Signaling Is a Common Factor Driving Streptococcus pneumoniae and Influenza A Virus Shedding and Transmission.mBio. 2021 Feb 16;12(1):e03589-20. doi: 10.1128/mBio.03589-20. mBio. 2021. PMID: 33593970 Free PMC article.
-
The Yin and Yang of Pneumolysin During Pneumococcal Infection.Front Immunol. 2022 Apr 22;13:878244. doi: 10.3389/fimmu.2022.878244. eCollection 2022. Front Immunol. 2022. PMID: 35529870 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

