Anti-oxidative Amino Acid L-ergothioneine Modulates the Tumor Microenvironment to Facilitate Adjuvant Vaccine Immunotherapy
- PMID: 31019508
- PMCID: PMC6458301
- DOI: 10.3389/fimmu.2019.00671
Anti-oxidative Amino Acid L-ergothioneine Modulates the Tumor Microenvironment to Facilitate Adjuvant Vaccine Immunotherapy
Abstract
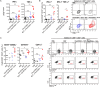
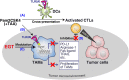
Cancer vaccines consist of a tumor-associated antigen (TAA) and adjuvant. These vaccines induce and activate proliferation of TAA-specific cytotoxic T lymphocytes (CTLs), suppressing tumor growth. The therapeutic efficacy of TAA-specific CTLs depends on the properties of tumor microenvironment. The environments make immunosuppressive by function of regulatory T cells and tumor-associated myeloid cells; thus, regulation of these cells is important for successful cancer immunotherapy. We report here that L-ergothioneine (EGT) with the adjuvant Toll-like receptor 2 (TLR2) ligand modulated suppressive microenvironments to be immune-enhancing. EGT did not augment DC-mediated CTL priming or affect CTL activation in draining lymph node and spleen. However, EGT decreased the immuno-suppressive function of tumor-associated macrophages (TAMs). TLR2 stimulation accompanied with EGT administration downregulated expression of PD-L1, CSF-1R, arginase-1, FAS ligand, and TRAIL in TAMs, reflecting reduction of CTL suppression. An anti-oxidative thiol-thione residue of EGT was essential to dampening CTL suppression. The effect was specific to the thiol-thione residue of EGT because no effect was observed with another anti-oxidant N-acetyl-L-cysteine (NAC). A CTL-suppressive environment made by TLR2 is relieved to be improved by the addition of EGT, which may ameliorate the efficacy of vaccine immunotherapy.
Keywords: L-ergothioneine; cytotoxic T lymphocyte; toll-like receptor; tumor microenvironment; tumor-associated macrophage.
Figures





References
-
- Ugel S, Mandruzzato S, Bronte V, Ugel S, De Sanctis F, Mandruzzato S, et al. Tumor-induced myeloid deviation : when myeloid-derived suppressor cells meet tumor- associated macrophages Find the latest version : tumor-induced myeloid deviation : when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. (2015) 125:3365–76. 10.1172/JCI80006.The - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

