First-line afatinib for the treatment of EGFR mutation-positive non-small-cell lung cancer in the 'real-world' clinical setting
- PMID: 31019567
- PMCID: PMC6466470
- DOI: 10.1177/1758835919836374
First-line afatinib for the treatment of EGFR mutation-positive non-small-cell lung cancer in the 'real-world' clinical setting
Abstract
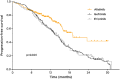
Afatinib is an ErbB family blocker that is approved for the treatment of epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer (NSCLC). Pivotal randomized clinical studies demonstrated that afatinib significantly prolonged progression-free survival compared with platinum-based chemotherapy (LUX-Lung 3, LUX-Lung 6), and with gefitinib (LUX-Lung 7), with manageable side effects. However, these results were derived from controlled studies conducted in selected patients and are not necessarily representative of real-world use of afatinib. To gain a broader understanding of the effectiveness and safety of first-line afatinib, we have undertaken a literature review of real-world studies that have assessed its use in a variety of patient populations. We focused on patients with uncommon EGFR mutations, brain metastases, or those of advanced age, as these patients are often excluded from clinical studies but are regularly seen in routine clinical practice. The available real-world studies suggest that afatinib has clinical activity, and is tolerable, in diverse patient populations in an everyday clinical practice setting. Moreover, consistent with LUX-Lung 7, several real-world comparative studies indicate that afatinib might confer better efficacy than first-generation EGFR tyrosine kinase inhibitors. Tolerability-guided dose adjustment, undertaken in 21-68% of patients in clinical practice, did not appear to reduce the efficacy of afatinib. Taken together, these findings provide further support for the use of afatinib as a treatment option in patients with EGFR mutation-positive NSCLC.
Keywords: EGFR tyrosine kinase inhibitors; NSCLC; afatinib; brain metastases; real-world; uncommon mutations.
Conflict of interest statement
Conflict of interest statement: KP has received personal fees from Boehringer Ingelheim, Clovis, Eli Lilly, Hanmi, Kyowa Hakko Kirin, Ono, Novartis, and Roche; and research funding from AstraZeneca. IO has received honoraria from Boehringer Ingelheim, AstraZeneca, Taiho Pharmaceuticals, Ono Pharmaceuticals, Merck Sharpe and Dohme, Lilly, Bristol-Myers Squibb, Chugai Pharma; and research funding from Boehringer Ingelheim. JY has received personal fees from Boehringer Ingelheim, AstraZeneca, Roche, Chugai, Eli Lilly, Clovis, Pfizer, Novartis, Merck Sharpe and Dohme, Astellas, Bayer, Innopharma, and Celgene; and research grants from Boehringer Ingelheim and AstraZeneca. DL reports no conflicts of interest.
Figures


References
-
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–742. - PubMed
-
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–246. - PubMed
-
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015; 26: 1883–1889. - PubMed
-
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–957. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

