Application of new biotechnologies for improvements in swine nutrition and pork production
- PMID: 31019685
- PMCID: PMC6474057
- DOI: 10.1186/s40104-019-0337-6
Application of new biotechnologies for improvements in swine nutrition and pork production
Abstract
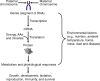
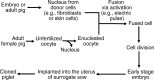
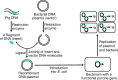
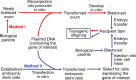
Meeting the increasing demands for high-quality pork protein requires not only improved diets but also biotechnology-based breeding to generate swine with desired production traits. Biotechnology can be classified as the cloning of animals with identical genetic composition or genetic engineering (via recombinant DNA technology and gene editing) to produce genetically modified animals or microorganisms. Cloning helps to conserve species and breeds, particularly those with excellent biological and economical traits. Recombinant DNA technology combines genetic materials from multiple sources into single cells to generate proteins. Gene (genome) editing involves the deletion, insertion or silencing of genes to produce: (a) genetically modified pigs with important production traits; or (b) microorganisms without an ability to resist antimicrobial substances. Current gene-editing tools include the use of zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), or clustered regularly interspaced short palindromic repeats-associated nuclease-9 (CRISPR/Cas9) as editors. ZFN, TALEN, or CRISPR/Cas9 components are delivered into target cells through transfection (lipid-based agents, electroporation, nucleofection, or microinjection) or bacteriophages, depending on cell type and plasmid. Compared to the ZFN and TALEN, CRISPR/Cas9 offers greater ease of design and greater flexibility in genetic engineering, but has a higher frequency of off-target effects. To date, genetically modified pigs have been generated to express bovine growth hormone, bacterial phytase, fungal carbohydrases, plant and C. elagan fatty acid desaturases, and uncoupling protein-1; and to lack myostatin, α-1,3-galactosyltransferase, or CD163 (a cellular receptor for the "blue ear disease" virus). Biotechnology holds promise in improving the efficiency of swine production and developing alternatives to antibiotics in the future.
Keywords: Biotechnology; Disease; Growth; Health; Meat production; Swine.
Conflict of interest statement
This article reviews published studies and does not require the approval of animal use or consent to participate.All authors read and approved the final manuscript.The authors declare that they have no competing interests.
Figures



References
-
- Boyd C, Cady R. A 50-year comparison of the carbon footprint of the U.S. swine herd: 1959–2009. London: Camco; 2012. pp. 1–29.
-
- Wu G. Principles of animal nutrition. Boca Raton: CRC Press; 2018.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

