A Review of Structural and Biomechanical Changes in the Cornea in Aging, Disease, and Photochemical Crosslinking
- PMID: 31019909
- PMCID: PMC6459081
- DOI: 10.3389/fbioe.2019.00066
A Review of Structural and Biomechanical Changes in the Cornea in Aging, Disease, and Photochemical Crosslinking
Abstract
The study of corneal biomechanics is motivated by the tight relationship between biomechanical properties and visual function within the ocular system. For instance, variation in collagen fibril alignment and non-enzymatic crosslinks rank high among structural factors which give rise to the cornea's particular shape and ability to properly focus light. Gradation in these and other factors engender biomechanical changes which can be quantified by a wide variety of techniques. This review summarizes what is known about both the changes in corneal structure and associated changes in corneal biomechanical properties in aging, keratoconic, and photochemically crosslinked corneas. In addition, methods for measuring corneal biomechanics are discussed and the topics are related to both clinical studies and biomechanical modeling simulations.
Keywords: aging; cornea; cornea biomechanical properties; crosslinking; crosslinking (CXL) corneal collagen; keratoconus.
Figures

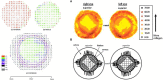

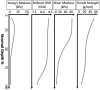

References
-
- Alvarado J., Murphy C., Juster R. (1983). Age-related changes in the basement membrane of the human corneal epithelium. Invest. Ophthalmol. Vis. Sci. 24, 1015–1028. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

