Polyphosphates and Complement Activation
- PMID: 31019911
- PMCID: PMC6458250
- DOI: 10.3389/fmed.2019.00067
Polyphosphates and Complement Activation
Abstract
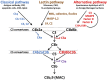
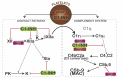
To sustain life in environments that are fraught with risks of life-threatening injury, organisms have developed innate protective strategies such that the response to wounds is rapid and localized, with the simultaneous recruitment of molecular, biochemical, and cellular pathways that limit bleeding and eliminate pathogens and damaged host cells, while promoting effective healing. These pathways are both coordinated and tightly regulated, as their over- or under-activation may lead to inadequate healing, disease, and/or demise of the host. Recent advances in our understanding of coagulation and complement, a key component of innate immunity, have revealed an intriguing linkage of the two systems. Cell-secreted polyphosphate promotes coagulation, while dampening complement activation, discoveries that are providing insights into disease mechanisms and suggesting novel therapeutic strategies.
Keywords: C1-esterase inhibitor; age-related macular degeneration; coagulation; inflammation; innate immunity; membrane attack complex; mouse models; thrombosis.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

