Structural brain anomalies in patients with FOXG1 syndrome and in Foxg1+/- mice
- PMID: 31019990
- PMCID: PMC6469254
- DOI: 10.1002/acn3.735
Structural brain anomalies in patients with FOXG1 syndrome and in Foxg1+/- mice
Abstract
Objective: FOXG1 syndrome is a rare neurodevelopmental disorder associated with heterozygous FOXG1 variants or chromosomal microaberrations in 14q12. The study aimed at assessing the scope of structural cerebral anomalies revealed by neuroimaging to delineate the genotype and neuroimaging phenotype associations.
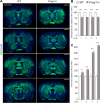
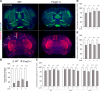
Methods: We compiled 34 patients with a heterozygous (likely) pathogenic FOXG1 variant. Qualitative assessment of cerebral anomalies was performed by standardized re-analysis of all 34 MRI data sets. Statistical analysis of genetic, clinical and neuroimaging data were performed. We quantified clinical and neuroimaging phenotypes using severity scores. Telencephalic phenotypes of adult Foxg1+/- mice were examined using immunohistological stainings followed by quantitative evaluation of structural anomalies.
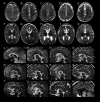
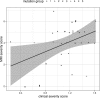
Results: Characteristic neuroimaging features included corpus callosum anomalies (82%), thickening of the fornix (74%), simplified gyral pattern (56%), enlargement of inner CSF spaces (44%), hypoplasia of basal ganglia (38%), and hypoplasia of frontal lobes (29%). We observed a marked, filiform thinning of the rostrum as recurrent highly typical pattern of corpus callosum anomaly in combination with distinct thickening of the fornix as a characteristic feature. Thickening of the fornices was not reported previously in FOXG1 syndrome. Simplified gyral pattern occurred significantly more frequently in patients with early truncating variants. Higher clinical severity scores were significantly associated with higher neuroimaging severity scores. Modeling of Foxg1 heterozygosity in mouse brain recapitulated the associated abnormal cerebral morphology phenotypes, including the striking enlargement of the fornix.
Interpretation: Combination of specific corpus callosum anomalies with simplified gyral pattern and hyperplasia of the fornices is highly characteristic for FOXG1 syndrome.
Conflict of interest statement
Nothing to report.
Figures
References
-
- Mencarelli MA, Kleefstra T, Katzaki E, et al. 14q12 Microdeletion syndrome and congenital variant of Rett syndrome. Eur J Med Genet 2009;52:148–152. - PubMed
-
- Philippe C, Amsallem D, Francannet C, et al. Phenotypic variability in Rett syndrome associated with FOXG1 mutations in females. J Med Genet 2010;47:59–65. - PubMed
-
- De Filippis R, Pancrazi L, Bjørgo K, et al. Expanding the phenotype associated with FOXG1 mutations and in vivo FoxG1 chromatin‐binding dynamics. Clin Genet 2012;82(4):395–403. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

