Immunotherapy with ponezumab for probable cerebral amyloid angiopathy
- PMID: 31020004
- PMCID: PMC6469253
- DOI: 10.1002/acn3.761
Immunotherapy with ponezumab for probable cerebral amyloid angiopathy
Abstract
Objective: Cerebral amyloid angiopathy (CAA) is caused by cerebrovascular deposition of β-amyloid fragments leading to cerebrovascular dysfunction and other brain injuries. This phase 2, randomized, double-blind trial in patients with probable CAA assessed the efficacy and safety of ponezumab, a novel monoclonal antibody against Aβ 1-40.
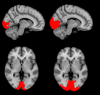
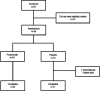
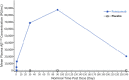
Methods: Thirty-six participants aged 55-80 years with probable CAA received intravenous placebo (n = 12) or ponezumab (n = 24). The change from baseline to Days 2 and 90 in cerebrovascular reactivity (CVR) was measured in the visual cortex as the natural log of the rising slope of the BOLD fMRI response to a visual stimulus. Safety and tolerability were also assessed.
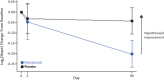
Results: The mean change from baseline to Day 90 was 0.817 (ponezumab) and 0.958 (placebo): a mean ratio of 0.852 (90% CI 0.735-0.989) representing a trend towards reduced CVR in the ponezumab group. This trend was not present at Day 2. There was one asymptomatic occurrence of amyloid-related imaging abnormality-edema in the ponezumab group. The total number of new cerebral microbleeds from baseline to day 90 did not differ between groups. The ponezumab group had a participant with nonfatal new cerebral hemorrhage with aphasia and a participant with subdural hemorrhage that site investigators deemed to be nondrug related. In the placebo group one participant had a fatal intracerebral hemorrhage and one participant had migraine with aura.
Interpretation: Ponezumab was safe and well-tolerated. The ponezumab group showed a trend towards treatment effect at Day 90 that was opposite to the hypothesized direction. The prespecified efficacy criteria were thus not met.
Conflict of interest statement
Claire Leurent, James A. Goodman, Yao Zhang, Ping He, Monica Lindsay, Linda Frattura, U. S. Sohur, and Martin M. Bednar were employees of Pfizer. Steven M. Greenberg served as a consultant to Pfizer and GlaxoSmithKline and on safety monitoring committees for amyloid immunotherapy studies conducted by Roche, Biogen, and DIAN‐TU. Massachusetts General Hospital participated in this study under a Clinical Research Support Agreement with Pfizer. Anand Viswanathan served on safety monitoring committees for amyloid immunotherapy studies conducted by Roche.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
