Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy
- PMID: 31020810
- PMCID: PMC6711418
- DOI: 10.1002/jcsm.12434
Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy
Abstract
Background: Skeletal muscle atrophy is defined as a reduction of muscle mass caused by excessive protein degradation. However, the development of therapeutic interventions is still in an early stage. Although glucagon-like peptide-1 receptor (GLP-1R) agonists, such as exendin-4 (Ex-4) and dulaglutide, are widely used for the treatment of diabetes, their effects on muscle pathology are unknown. In this study, we investigated the therapeutic potential of GLP-1R agonist for muscle wasting and the mechanisms involved.
Methods: Mouse C2C12 myotubes were used to evaluate the in vitro effects of Ex-4 in the presence or absence of dexamethasone (Dex) on the regulation of the expression of muscle atrophic factors and the underlying mechanisms using various pharmacological inhibitors. In addition, we investigated the in vivo therapeutic effect of Ex-4 in a Dex-induced mouse muscle atrophy model (20 mg/kg/day i.p.) followed by injection of Ex-4 (100 ng/day i.p.) for 12 days and chronic kidney disease (CKD)-induced muscle atrophy model. Furthermore, we evaluated the effect of a long-acting GLP-1R agonist by treatment of dulaglutide (1 mg/kg/week s.c.) for 3 weeks, in DBA/2J-mdx mice, a Duchenne muscular dystrophy model.
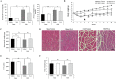
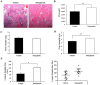
Results: Ex-4 suppressed the expression of myostatin (MSTN) and muscle atrophic factors such as F-box only protein 32 (atrogin-1) and muscle RING-finger protein-1 (MuRF-1) in Dex-treated C2C12 myotubes. The suppression effect was via protein kinase A and protein kinase B signalling pathways through GLP-1R. In addition, Ex-4 treatment inhibited glucocorticoid receptor (GR) translocation by up-regulating the proteins of GR inhibitory complexes. In a Dex-induced muscle atrophy model, Ex-4 ameliorated muscle atrophy by suppressing muscle atrophic factors and enhancing myogenic factors (MyoG and MyoD), leading to increased muscle mass and function. In the CKD muscle atrophy model, Ex-4 also increased muscle mass, myofiber size, and muscle function. In addition, treatment with a long-acting GLP-1R agonist, dulaglutide, recovered muscle mass and function in DBA/2J-mdx mice.
Conclusions: GLP-1R agonists ameliorate muscle wasting by suppressing MSTN and muscle atrophic factors and enhancing myogenic factors through GLP-1R-mediated signalling pathways. These novel findings suggest that activating GLP-1R signalling may be useful for the treatment of atrophy-related muscular diseases.
Keywords: Chronic kidney disease; Dexamethasone; Duchenne muscular dystrophy; GLP-1R agonists; Glucocorticoid receptor; Skeletal muscle atrophy.
© 2019 The Authors Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
Y.H.H., J.H.L., K.W.J., C.S.C., and H.‐S.J. declare that they have no conflict of interest.
Figures





References
-
- Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 2015;14:58–74. - PubMed
-
- Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 2010;91:1128s–1132s. - PubMed
-
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. - PubMed
-
- Williams A, Sun X, Fischer JE, Hasselgren PO. The expression of genes in the ubiquitin‐proteasome proteolytic pathway is increased in skeletal muscle from patients with cancer. Surgery 1999;126:744–749, discussion 749‐50, 750. - PubMed
-
- Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

