Heterogeneity of the action potential duration is required for sustained atrial fibrillation
- PMID: 31021331
- PMCID: PMC6629142
- DOI: 10.1172/jci.insight.128765
Heterogeneity of the action potential duration is required for sustained atrial fibrillation
Abstract
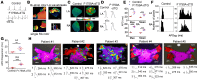
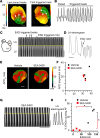
Atrial fibrillation (AF) is the most common cardiac arrhythmia and accounts for substantial morbidity and mortality. Recently, we created a mouse model with spontaneous and sustained AF caused by a mutation in the NaV1.5 channel (F1759A) that enhances persistent Na+ current, thereby enabling the investigation of molecular mechanisms that cause AF and the identification of novel treatment strategies. The mice have regional heterogeneity of action potential duration of the atria similar to observations in patients with AF. In these mice, we found that the initiation and persistence of the rotational reentrant AF arrhythmias, known as spiral waves or rotors, were dependent upon action potential duration heterogeneity. The centers of the rotors were localized to regions of greatest heterogeneity of the action potential duration. Pharmacologically attenuating the action potential duration heterogeneity reduced both spontaneous and pacing-induced AF. Computer-based simulations also demonstrated that the action potential duration heterogeneity is sufficient to generate rotors that manifest as AF. Taken together, these findings suggest that action potential duration heterogeneity in mice and humans is one mechanism by which AF is initiated and that reducing action potential duration heterogeneity can lessen the burden of AF.
Keywords: Arrhythmias; Cardiology; Cell Biology; Ion channels; Mouse models.
Conflict of interest statement
Figures



Similar articles
-
Loss of insulin signaling may contribute to atrial fibrillation and atrial electrical remodeling in type 1 diabetes.Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7990-8000. doi: 10.1073/pnas.1914853117. Epub 2020 Mar 20. Proc Natl Acad Sci U S A. 2020. PMID: 32198206 Free PMC article.
-
Atrial fibrillation and electrophysiology in transgenic mice with cardiac-restricted overexpression of FKBP12.Am J Physiol Heart Circ Physiol. 2019 Feb 1;316(2):H371-H379. doi: 10.1152/ajpheart.00486.2018. Epub 2018 Nov 30. Am J Physiol Heart Circ Physiol. 2019. PMID: 30499712 Free PMC article.
-
Electrophysiologic and molecular mechanisms of a frameshift NPPA mutation linked with familial atrial fibrillation.J Mol Cell Cardiol. 2019 Jul;132:24-35. doi: 10.1016/j.yjmcc.2019.05.004. Epub 2019 May 8. J Mol Cell Cardiol. 2019. PMID: 31077706 Free PMC article.
-
Regulatory role of cardiomyocyte metabolism via AMPK activation in modulating atrial structural, contractile, and electrical properties following atrial fibrillation.Can J Physiol Pharmacol. 2021 Jan;99(1):36-41. doi: 10.1139/cjpp-2020-0313. Epub 2020 Oct 13. Can J Physiol Pharmacol. 2021. PMID: 33049144 Review.
-
Mechanisms of atrial fibrillation: rotors, ionic determinants, and excitation frequency.Cardiol Clin. 2014 Nov;32(4):495-506. doi: 10.1016/j.ccl.2014.07.001. Epub 2014 Oct 23. Cardiol Clin. 2014. PMID: 25443232 Free PMC article. Review.
Cited by
-
Fibroblast growth factor homologous factors tune arrhythmogenic late NaV1.5 current in calmodulin binding-deficient channels.JCI Insight. 2020 Oct 2;5(19):e141736. doi: 10.1172/jci.insight.141736. JCI Insight. 2020. PMID: 32870823 Free PMC article.
-
Quantitative 3D electron microscopy characterization of mitochondrial structure, mitophagy, and organelle interactions in murine atrial fibrillation.J Struct Biol. 2024 Sep;216(3):108110. doi: 10.1016/j.jsb.2024.108110. Epub 2024 Jul 14. J Struct Biol. 2024. PMID: 39009246 Free PMC article.
-
Glucagon-Like Peptide-1 Protects Against Atrial Fibrillation and Atrial Remodeling in Type 2 Diabetic Mice.JACC Basic Transl Sci. 2023 Mar 15;8(8):922-936. doi: 10.1016/j.jacbts.2023.01.005. eCollection 2023 Aug. JACC Basic Transl Sci. 2023. PMID: 37719430 Free PMC article.
-
Challenges Associated with Interpreting Mechanisms of AF.Arrhythm Electrophysiol Rev. 2020 Feb 12;8(4):273-284. doi: 10.15420/aer.2019.08. Arrhythm Electrophysiol Rev. 2020. PMID: 32685158 Free PMC article. Review.
-
Theoretical Models and Computational Analysis of Action Potential Dispersion for Cardiac Arrhythmia Risk Stratification.Front Cardiovasc Med. 2021 Mar 5;8:649489. doi: 10.3389/fcvm.2021.649489. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33748198 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

