Palladium/Norbornene Cooperative Catalysis
- PMID: 31021606
- PMCID: PMC7075350
- DOI: 10.1021/acs.chemrev.9b00079
Palladium/Norbornene Cooperative Catalysis
Abstract
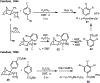
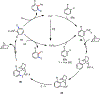
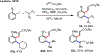
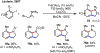
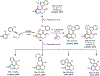
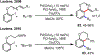
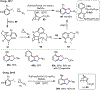
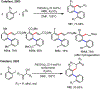
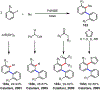
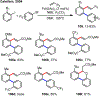
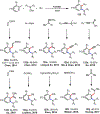
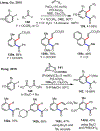
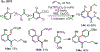
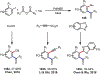
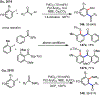
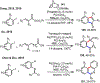
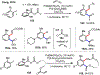
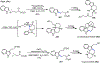
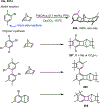
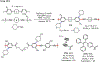
Palladium/norbornene cooperative catalysis has emerged as a distinct approach to construct polyfunctionalized arenes from readily available starting materials. This Review provides a comprehensive overview of this field, including the early stoichiometric investigations, catalytic reaction developments, as well as the applications in the syntheses of bioactive compounds and polymers. The section of catalytic reactions is divided into two parts according to the reaction initiation mode: Pd(0)-initiated reactions and Pd(II)-initiated reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




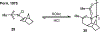
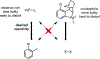
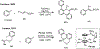

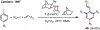

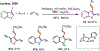






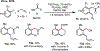


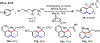
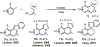


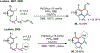

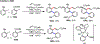









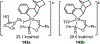













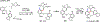

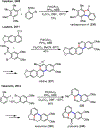
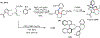

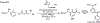
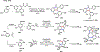

References
-
- Taylor RD; MacCoss M; Lawson ADG ″Rings in Drugs″. J. Med. Chem 2014, 57, 5845–5859. - PubMed
-
- Tsuji J Palladium Reagents and Catalysts: New Perspectives for the 21st Century; John Wiley & Sons, Ltd, 2005.
-
- Terrier F Modern Nucleophilic Aromatic Substitution; Wiley-VCH Verlag GmbH & Co. KGaA, 2013.
-
- Negishi E.-i.; De Meijere A Handbook of Organopalladium Chemistry for Organic Synthesis; John Wiley & Sons, Inc., 2003.
-
- Buncel E; Dust JM; Terrier F ″Rationalizing the Regioselectivity in Polynitroarene Anionic σ-Adduct Formation. Relevance to Nucleophilic Aromatic Substitution″. Chem. Rev 1995, 95, 2261–2280.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

