Electrospinning Optimization of Eudragit E PO with and without Chlorpheniramine Maleate Using a Design of Experiment Approach
- PMID: 31021642
- PMCID: PMC6549214
- DOI: 10.1021/acs.molpharmaceut.9b00159
Electrospinning Optimization of Eudragit E PO with and without Chlorpheniramine Maleate Using a Design of Experiment Approach
Abstract
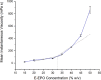
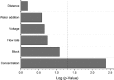
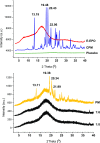
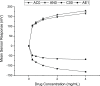
Electrospinning is increasingly becoming a viable means of producing drug delivery vehicles for oral delivery, particularly as issues of manufacturing scalability are being addressed. In this study, electrospinning is explored as a taste-masking manufacturing technology for bitter drugs. The taste-masking polymer Eudragit E PO (E-EPO) was electrospun, guided by a quality by design approach. Using a design of experiment, factors influencing the production of smooth fibers were investigated. Polymer concentration, solvent composition, applied voltage, flow rate, and gap distance were the parameters examined. Of these, polymer concentration was shown to be the only statistically significant factor within the ranges studied ( p-value = 0.0042). As the concentration increased, smoother fibers were formed, coupled with an increase in fiber diameter. E-EPO (35% w/v) was identified as the optimum concentration for smooth fiber production. The optimized processing conditions identified were a gap distance of 175 mm, an applied voltage of between 15 and 20 kV, and a flow rate of 1 mL/h. Using this knowledge, the production optimization of electrospun E-EPO with chlorpheniramine maleate (CPM), a bitter antihistamine drug, was explored. The addition of CPM in drug loads of 1:6 up to 1:10 CPM/E-EPO yielded smooth fibers that were electrospun under conditions similar to placebo fibers. Solid-state characterization showed CPM to be molecularly dispersed in E-EPO. An electronic tasting system, or E-tongue, indicated good taste-masking performance as compared to the equivalent physical mixtures. This study therefore describes a means of producing, optimizing, and assessing the performance of electrospun taste-masked fibers as a novel approach to the formulation of CPM and potentially other bitter drug substances.
Keywords: DoE; E-tongue; Eudragit E PO; chlorpheniramine maleate; electrospinning; taste-masking.
Conflict of interest statement
The authors declare the following competing financial interest(s): This work was financially supported by the Medical Research Council, London, UK; iCASE Award No. 170156 and Pfizer Ltd, Sandwich, UK; Award No. 173803.
Figures












References
-
- Brako F.; Raimi-Abraham B.; Mahalingam S.; Craig D. Q. M.; Edirisinghe M. Making Nanofibres of Mucoadhesive Polymer Blends for Vaginal Therapies. Eur. Polym. J. 2015, 70, 186–196. 10.1016/j.eurpolymj.2015.07.006. - DOI
-
- Wu Y.-H.; Yu D.-G.; Li X.-Y.; Diao A.-H.; Illangakoon U. E.; Williams G. R. Fast-Dissolving Sweet Sedative Nanofiber Membranes. J. Mater. Sci. 2015, 50, 3604–3613. 10.1007/s10853-015-8921-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

