Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization
- PMID: 31021652
- PMCID: PMC6529341
- DOI: 10.1096/fj.201802580R
Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization
Abstract
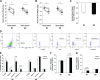
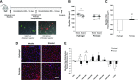
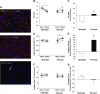
Conditions of extended bed rest and limb immobilization can initiate rapid and significant loss of skeletal muscle mass and function. Physical rehabilitation is standard practice following a period of disuse, yet mobility may be severely compromised, and recovery is commonly delayed or incomplete in special populations. Thus, a novel approach toward recovery of muscle mass is highly desired. Pericytes [neuron-glial antigen 2 (NG2)+CD31-CD45- (Lineage- [Lin-]) and CD146+Lin-] demonstrate capacity to facilitate muscle repair, yet the ability to enhance myofiber growth following disuse is unknown. In the current study, 3-4-mo-old mice were unilaterally immobilized for 14 d (IM) or immobilized for 14 d followed by 14 d of remobilization (RE). Flow cytometry and targeted gene expression analyses were completed to assess pericyte quantity and function following IM and RE. In addition, a transplantation study was conducted to assess the impact of pericytes on recovery. Results from targeted analyses suggest minimal impact of disuse on pericyte gene expression, yet NG2+Lin- pericyte quantity is reduced following IM (P < 0.05). Remarkably, pericyte transplantation recovered losses in myofiber cross-sectional area and the capillary-to-fiber ratio following RE, whereas deficits remained with vehicle alone (P = 0.01). These findings provide the first evidence that pericytes effectively rehabilitate skeletal muscle mass following disuse atrophy.-Munroe, M., Dvoretskiy, S., Lopez, A., Leong, J., Dyle, M. C., Kong, H., Adams, C. M., Boppart, M. D. Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization.
Keywords: capillary; disuse atrophy; muscle growth; rehabilitation; stem cells.
Conflict of interest statement
The authors thank Dr. Barbara Pilas and the Flow Cytometry Center at the Roy J. Carver Biotechnology Center (University of Illinois–Urbana-Champaign) for advice and assistance. Research reported in this publication was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the U.S. National Institutes of Health (NIH) under award number R01AR072735 (to M.D.B.), National Heart, Lung, and Blood Institute (NHLBI) under award number R21HL131469 (to H.K. and M.D.B.), and a University of Illinois–Urbana-Champaign (UIUC) Research Board Grant (to M.D.B.). M.M. was supported by a UIUC Dissertation Completion Award. S.D. was supported by National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the NIH under award number T32EB019944 and an American College of Sports Medicine National Aeronautics and Space Administration (NASA) Space Physiology Research Grant (18-00664). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Figures



References
-
- Dirks M. L., Wall B. T., Nilwik R., Weerts D. H., Verdijk L. B., van Loon L. J. (2014) Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J. Nutr. 144, 1196–1203 - PubMed
-
- Hvid L. G., Suetta C., Aagaard P., Kjaer M., Frandsen U., Ørtenblad N. (2013) Four days of muscle disuse impairs single fiber contractile function in young and old healthy men. Exp. Gerontol. 48, 154–161 - PubMed
-
- Wall B. T., Snijders T., Senden J. M., Ottenbros C. L., Gijsen A. P., Verdijk L. B., van Loon L. J. (2013) Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J. Clin. Endocrinol. Metab. 98, 4872–4881 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

