Sodium chloride primes JA-independent defense against Spodoptera litura (Fabricius) larvae in Arabidopsis thaliana
- PMID: 31021696
- PMCID: PMC6619998
- DOI: 10.1080/15592324.2019.1607466
Sodium chloride primes JA-independent defense against Spodoptera litura (Fabricius) larvae in Arabidopsis thaliana
Abstract
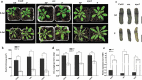
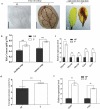
Priming for better defense performance is an important strategy in acclimation to the ever-changing environment. In the present study, defense priming induced by sodium chloride at the seedling stage significantly increased the expression of defense gene VSP2, the content of total glucosinolates and the level of the reactive oxygen species in mature Arabidopsis thaliana plants after transferred into the stress-free environment. The previously primed plants could effectively resist the feeding of Spodoptera litura (Fabricius) larvae. Salt-priming enhanced defense of Arabidopsis plants in the absence of either MYC2 or AOS, which encodes a critical transcription factor in JA-signaling and an important enzyme in JA biosynthesis, respectively. Our results supported the JA-independent defense primed by sodium chloride, as well as the elevated ROS and glucosinolate level in primed plants. In addition, the feasibility of using mild salt-priming to improve crop performance in field was proposed.
Keywords: Sodium chloride; defense priming; herbivory; jasmonic acid pathway.
Figures

Similar articles
-
CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis.Plant Physiol. 2012 Jul;159(3):1159-75. doi: 10.1104/pp.112.198150. Epub 2012 May 8. Plant Physiol. 2012. PMID: 22570470 Free PMC article.
-
The Ca2+ Channel CNGC19 Regulates Arabidopsis Defense Against Spodoptera Herbivory.Plant Cell. 2019 Jul;31(7):1539-1562. doi: 10.1105/tpc.19.00057. Epub 2019 May 10. Plant Cell. 2019. PMID: 31076540 Free PMC article.
-
The Arabidopsis immune regulator SRFR1 dampens defences against herbivory by Spodoptera exigua and parasitism by Heterodera schachtii.Mol Plant Pathol. 2016 May;17(4):588-600. doi: 10.1111/mpp.12304. Epub 2015 Nov 6. Mol Plant Pathol. 2016. PMID: 26310916 Free PMC article.
-
Varied response of Spodoptera littoralis against Arabidopsis thaliana with metabolically engineered glucosinolate profiles.Plant Physiol Biochem. 2012 Jan;50(1):72-8. doi: 10.1016/j.plaphy.2011.07.014. Epub 2011 Jul 29. Plant Physiol Biochem. 2012. PMID: 21835629
-
Plant-Insect Bioassay for Testing Arabidopsis Resistance to the Generalist Herbivore Spodoptera littoralis.Methods Mol Biol. 2020;2085:69-78. doi: 10.1007/978-1-0716-0142-6_5. Methods Mol Biol. 2020. PMID: 31734917
Cited by
-
A New Species in Pseudophialophora From Wild Rice and Beneficial Potential.Front Microbiol. 2022 Mar 11;13:845104. doi: 10.3389/fmicb.2022.845104. eCollection 2022. Front Microbiol. 2022. PMID: 35359723 Free PMC article.
References
-
- Chen K, Arora R. Priming memory invokes seed stress-tolerance. Environ Exp Bot. 2013;94(6):33–45. doi:10.1016/j.envexpbot.2012.03.005. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
