Effect of Ketoprofen and ATB-352 on the Immature Human Intestine: Identification of Responders and Non-responders
- PMID: 31022092
- PMCID: PMC6510328
- DOI: 10.1097/MPG.0000000000002308
Effect of Ketoprofen and ATB-352 on the Immature Human Intestine: Identification of Responders and Non-responders
Abstract
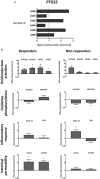
Background and objective: The use of nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a broad spectrum of life-threatening adverse effects on the immature gastrointestinal tract. NSAID derivatives exploiting the beneficial effects of biologically active gases, such as hydrogen sulfide (H2S), have been developed. Herein, we determined the effects of ketoprofen and ATB-352, a H2S-releasing ketoprofen derivative, on selected metabolic pathways previously identified to be significantly altered by indomethacin in the human immature intestine.
Methods: Ketoprofen and ATB-352 were tested on human mid-gestation small intestinal explants maintained in a serum-free organ culture system for 48 hours. The expression levels of the representative genes involved in selected metabolic pathways were measured by real-time PCR after a treatment of 48 hours.
Results: Tested at a concentration that allows more than 80% inhibition of PGE2 production, ketoprofen was found to be less damaging than indomethacin at an equivalent dosage. However, based on the inducibility of cyclooxygenase-2 transcript expression, we were able to discriminate between responder individuals in which the deleterious effects observed with indomethacin were attenuated, and non-responder specimens in which the effects were similar to those observed with indomethacin. ATB-352 did not induce significant changes compared to ketoprofen on these metabolic pathways.
Conclusions: These results show less damaging effects of ketoprofen compared to indomethacin on the immature intestine and indicate that the intestinal response to this NSAID significantly varies between individuals. However, the results did not allow us to demonstrate a specific beneficial effect of H2S release in organ culture.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


References
-
- Berkman ND, Thorp JM, Jr, Lohr KN, et al. Tocolytic treatment for the management of preterm labor: a review of the evidence. Am J Obstet Gynecol 2003; 188:1648–1659. - PubMed
-
- Van Overmeire B, Chemtob S. The pharmacologic closure of the patent ductus arteriosus. Semin Fetal Neonatal Med 2005; 10:177–184. - PubMed
-
- Bjarnason I, Scarpignato C, Holmgren E, et al. Mechanisms of damage to the gastrointestinal tract from non-steroidal anti-inflammatory drugs. Gastroenterology 2018; 154:500–514. - PubMed
-
- Scheiman JM. NSAID-induced gastrointestinal injury: a focused update for clinicians. J Clin Gastroenterol 2016; 50:5–10. - PubMed
-
- Srinivasan A, De Cruz P. Review article: a practical approach to the clinical management of NSAID enteropathy. Scand J Gastroenterol 2017; 52:941–947. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

