Necrosis, apoptosis, necroptosis, three modes of action of dopaminergic neuron neurotoxins
- PMID: 31022188
- PMCID: PMC6483187
- DOI: 10.1371/journal.pone.0215277
Necrosis, apoptosis, necroptosis, three modes of action of dopaminergic neuron neurotoxins
Abstract
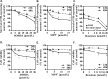
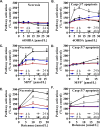
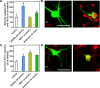
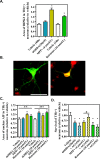
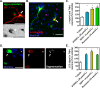
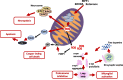
Most of the Parkinson's disease (PD) cases are sporadic, although several genes are directly related to PD. Several pathways are central in PD pathogenesis: protein aggregation linked to proteasomal impairments, mitochondrial dysfunctions and impairment in dopamine (DA) release. Here we studied the close crossing of mitochondrial dysfunction and aggregation of α-synuclein (α-syn) and in the extension in the dopaminergic neuronal death. Here, using rat primary cultures of mesencephalic neurons, we induced the mitochondrial impairments using "DA-toxins" (MPP+, 6OHDA, rotenone). We showed that the DA-Toxins induced dopaminergic cell death through different pathways: caspase-dependent cell death for 6OHDA; MPP+ stimulated caspase-independent cell death, and rotenone activated both pathways. In addition, a decrease in energy production and/or a development of oxidative stress were observed and were linked to α-syn aggregation with generation of Lewy body-like inclusions (found inside and outside the dopaminergic neurons). We demonstrated that any of induced mitochondrial disturbances and processes of death led to α-syn protein aggregation and finally to cell death. Our study depicts the cell death mechanisms taking place in in vitro models of Parkinson's disease and how mitochondrial dysfunctions is at the cross road of the pathologies of this disease.
Conflict of interest statement
This study was funded by internal funds of Neuro-Sys SAS. NC, MC, and AH are emplyees of this funder. PP is a member of its scientific board. This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

