Evaluation of enterotoxin gene expression and enterotoxin production capacity of the probiotic strain Bacillus toyonensis BCT-7112T
- PMID: 31022208
- PMCID: PMC6483178
- DOI: 10.1371/journal.pone.0214536
Evaluation of enterotoxin gene expression and enterotoxin production capacity of the probiotic strain Bacillus toyonensis BCT-7112T
Abstract
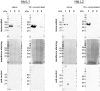
The aim of the present study was to evaluate the safety of the probiotic strain Bacillus toyonensis BCT-7112T (active ingredient of Toyocerin) in relation to the enterotoxins haemolysin BL (Hbl) and the non-haemolytic enterotoxin (Nhe) by performing a quantitative reverse transcription (RT) real-time polymerase chain reaction (PCR) and a Western blot assay. The expression levels of the enterotoxin genes hblA, hblD, nheA, nheB and nheC, determined by means of RT real-time PCR in B. toyonensis, were lower than those in B. cereus reference strains. No expression of hblC was detected. The Western blot assays of native and 25-fold concentrated supernatants from B. toyonensis, using monoclonal antibodies directed against the Hbl component L1 and the Nhe component NheB, showed weak bands. The NheC component was not detected in the native supernatant, but weakly in the 25-fold concentrated supernatant. According to the results of the present study, the enterotoxin expression and protein levels of B. toyonensis BCT-7112T were absent or clearly lower compared to the B. cereus reference strains. Thus, their ability to form functional enterotoxins can also be considered to be lower or unlikely compared to the B. cereus reference strains. This experimental approach can be implemented when studying the health and safety as well as harmlessness of probiotic microorganisms.
Conflict of interest statement
I have read the journal´s policy. G. Jimenez is an employee of Rubinum, S. A., that has a commercial interest in B. toyonensis BCT-7112T. This does not alter our adherence to PLOS ONE policies on sharing data and materials. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

