The protein elicitor Hrip1 enhances resistance to insects and early bolting and flowering in Arabidopsis thaliana
- PMID: 31022256
- PMCID: PMC6483360
- DOI: 10.1371/journal.pone.0216082
The protein elicitor Hrip1 enhances resistance to insects and early bolting and flowering in Arabidopsis thaliana
Abstract
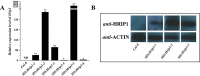
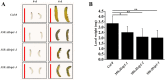
The elicitor Hrip1 isolated from necrotrophic fungus Alternaria tenuissima, could induce systemic acquired resistance in tobacco to enhance resistance to tobacco mosaic virus. In the present study, we found that the transgenic lines of Hrip1-overexpression in wild type (WT) Arabidopsis thaliana were more resistant to Spodoptera exigua and were early bolting and flowering than the WT. A profiling of transcription assay using digital gene expression profiling was used for transgenic and WT Arabidopsis thaliana. Differentially expressed genes including 40 upregulated and three downregulated genes were identified. In transgenic lines of Hrip1-overexpression, three genes related to jasmonate (JA) biosynthesis were significantly upregulated, and the JA level was found to be higher than WT. Two GDSL family members (GLIP1 and GLIP4) and pathogen-related gene, which participated in pathogen defense action, were upregulated in the transgenic line of Hrip1-overexpression. Thus, Hrip1 is involved in affecting the flower bolting time and regulating endogenous JA biosynthesis and regulatory network to enhance resistance to insect.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

