Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization
- PMID: 31022431
- PMCID: PMC7111479
- DOI: 10.1016/j.jconrel.2019.04.025
Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization
Abstract
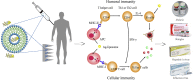
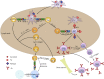
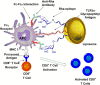
Liposomes are widely utilized as a carrier to improve therapeutic efficacy of agents thanks to their merits of high loading capacity, targeting delivery, reliable protection of agents, good biocompatibility, versatile structure modification and adjustable characteristics, such as size, surface charge, membrane flexibility and the agent loading mode. In particular, in recent years, through modification with immunopotentiators and targeting molecules, and in combination with innovative immunization devices, liposomes are rapidly developed as a multifunctional vaccine adjuvant-delivery system (VADS) that has a high capability in inducing desired immunoresponses, as they can target immune cells and even cellular organelles, engender lysosome escape, and promote Ag cross-presentation, thus enormously enhancing vaccination efficacy. Moreover, after decades of development, several products developed on liposome VADS have already been authorized for clinical immunization and are showing great advantages over conventional vaccines. This article describes in depth some critical issues relevant to the development of liposomes as a VADS, including principles underlying immunization, physicochemical properties of liposomes as the immunity-influencing factors, functional material modification to enhance immunostimulatory functions, the state-of-the-art liposome VADSs, as well as the marketed vaccines based on a liposome VADS. Therefore, this article provides a comprehensive reference to the development of novel liposome vaccines.
Keywords: Antigen cross-presentation; Cellular immunity; Immunoresponse; Mucosal vaccination; Pathogen-associated molecular pattern; Vaccine adjuvant-delivery system.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures









References
-
- Del Giudice G., Rappuoli R., Didierlaurent A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018;39:14–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

