The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine
- PMID: 31022557
- PMCID: PMC6527863
- DOI: 10.1016/j.biomaterials.2019.04.009
The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine
Abstract
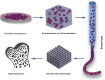
Recent advances in regenerative medicine have confirmed the potential to manufacture viable and effective tissue engineering 3D constructs comprising living cells for tissue repair and augmentation. Cell printing has shown promising potential in cell patterning in a number of studies enabling stem cells to be precisely deposited as a blueprint for tissue regeneration guidance. Such manufacturing techniques, however, face a number of challenges including; (i) post-printing cell damage, (ii) proliferation impairment and, (iii) poor or excessive final cell density deposition. The use of hydrogels offers one approach to address these issues given the ability to tune these biomaterials and subsequent application as vectors capable of delivering cell populations and as extrusion pastes. While stem cell-laden hydrogel 3D constructs have been widely established in vitro, clinical relevance, evidenced by in vivo long-term efficacy and clinical application, remains to be demonstrated. This review explores the central features of cell printing, cell-hydrogel properties and cell-biomaterial interactions together with the current advances and challenges in stem cell printing. A key focus is the translational hurdles to clinical application and how in vivo research can reshape and inform cell printing applications for an ageing population.
Keywords: 3D printing; Additive manufacturing; Biofabrication; Bioink; Bioprinting; Cell printing; Hydrogels.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures





Similar articles
-
The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells.Acta Biomater. 2017 Oct 1;61:41-53. doi: 10.1016/j.actbio.2017.08.005. Epub 2017 Aug 4. Acta Biomater. 2017. PMID: 28782725 Free PMC article.
-
Bioprinting stem cells: building physiological tissues one cell at a time.Am J Physiol Cell Physiol. 2020 Sep 1;319(3):C465-C480. doi: 10.1152/ajpcell.00124.2020. Epub 2020 Jul 8. Am J Physiol Cell Physiol. 2020. PMID: 32639873 Review.
-
Converging functionality: Strategies for 3D hybrid-construct biofabrication and the role of composite biomaterials for skeletal regeneration.Acta Biomater. 2021 Sep 15;132:188-216. doi: 10.1016/j.actbio.2021.03.008. Epub 2021 Mar 10. Acta Biomater. 2021. PMID: 33713862 Review.
-
Hybrid biofabrication of 3D osteoconductive constructs comprising Mg-based nanocomposites and cell-laden bioinks for bone repair.Bone. 2022 Jan;154:116198. doi: 10.1016/j.bone.2021.116198. Epub 2021 Sep 15. Bone. 2022. PMID: 34534709
-
Nanocomposite Clay-Based Bioinks for Skeletal Tissue Engineering.Methods Mol Biol. 2021;2147:63-72. doi: 10.1007/978-1-0716-0611-7_6. Methods Mol Biol. 2021. PMID: 32840811
Cited by
-
Optimising Bioprinting Nozzles through Computational Modelling and Design of Experiments.Biomimetics (Basel). 2024 Jul 29;9(8):460. doi: 10.3390/biomimetics9080460. Biomimetics (Basel). 2024. PMID: 39194439 Free PMC article. Review.
-
Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering.Gels. 2023 Mar 15;9(3):230. doi: 10.3390/gels9030230. Gels. 2023. PMID: 36975679 Free PMC article. Review.
-
3D printing of cell-delivery scaffolds for tissue regeneration.Regen Biomater. 2023 Mar 27;10:rbad032. doi: 10.1093/rb/rbad032. eCollection 2023. Regen Biomater. 2023. PMID: 37081861 Free PMC article. Review.
-
3D Bioprinting of Vascularized Tissues for in vitro and in vivo Applications.Front Bioeng Biotechnol. 2021 May 13;9:664188. doi: 10.3389/fbioe.2021.664188. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34055761 Free PMC article. Review.
-
Prospects and Challenges of Translational Corneal Bioprinting.Bioengineering (Basel). 2020 Jul 6;7(3):71. doi: 10.3390/bioengineering7030071. Bioengineering (Basel). 2020. PMID: 32640721 Free PMC article. Review.
References
-
- Kang H.-W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016;34:312–319. - PubMed
-
- Klebe R.J. Cytoscribing: a method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Exp. Cell Res. 1988;179:362–373. - PubMed
-
- V Murphy S., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. - PubMed
-
- Biggs M.J.P., Richards R.G., Gadegaard N., Wilkinson C.D.W., Oreffo R.O.C., Dalby M.J. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials. 2009;30:5094–5103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/R015651/1/MRC_/Medical Research Council/United Kingdom
- BB/P017711/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/LO21072/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L00609X/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

