Limitations to adaptive homeostasis in an hyperoxia-induced model of accelerated ageing
- PMID: 31022673
- PMCID: PMC6479762
- DOI: 10.1016/j.redox.2019.101194
Limitations to adaptive homeostasis in an hyperoxia-induced model of accelerated ageing
Abstract
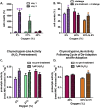
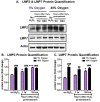
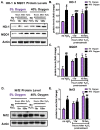
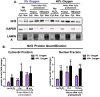
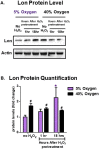
The Nrf2 signal transduction pathway plays a major role in adaptive responses to oxidative stress and in maintaining adaptive homeostasis, yet Nrf2 signaling undergoes a significant age-dependent decline that is still poorly understood. We used mouse embryonic fibroblasts (MEFs) cultured under hyperoxic conditions of 40% O2, as a model of accelerated ageing. Hyperoxia increased baseline levels of Nrf2 and multiple transcriptional targets (20S Proteasome, Immunoproteasome, Lon protease, NQO1, and HO-1), but resulted in loss of cellular ability to adapt to signaling levels (1.0 μM) of H2O2. In contrast, MEFs cultured at physiologically relevant conditions of 5% O2 exhibited a transient induction of Nrf2 Phase II target genes and stress-protective enzymes (the Lon protease and OXR1) following H2O2 treatment. Importantly, all of these effects have been seen in older cells and organisms. Levels of Two major Nrf2 inhibitors, Bach1 and c-Myc, were strongly elevated by hyperoxia and appeared to exert a ceiling on Nrf2 signaling. Bach1 and c-Myc also increase during ageing and may thus be the mechanism by which adaptive homeostasis is compromised with age.
Keywords: 20S proteasome; Adaptive homeostasis; Bach1; Hyperoxia; Immunoproteasome; Lon protease; NQO1; Nrf2; OXR1; c-Myc.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Davies J.M., Lowry C.V., Davies K.J. Transient adaptation to oxidative stress in yeast. Arch. Biochem. Biophys. 1995;317(1):1–6. - PubMed
-
- Grune T., Reinheckel T., Davies K. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11(7):526–534. - PubMed
-
- Grune T., Reinheckel T., Davies K.J. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J. Biol. Chem. 1996;271(26):15504–15509. - PubMed
-
- Grune T. Proteolysis in cultured liver epithelial cells during oxidative stress Role of the multicatalytic proteinase complex, proteasome. J. Biol. Chem. 1995;270(5):2344–2351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

