Quantitative analysis of the yeast pheromone pathway
- PMID: 31022772
- PMCID: PMC6684483
- DOI: 10.1002/yea.3395
Quantitative analysis of the yeast pheromone pathway
Abstract
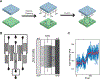
The pheromone response pathway of the yeast Saccharomyces cerevisiae is a well-established model for the study of G proteins and mitogen-activated protein kinase (MAPK) cascades. Our longstanding ability to combine sophisticated genetic approaches with established functional assays has provided a thorough understanding of signalling mechanisms and regulation. In this report, we compare new and established methods used to quantify pheromone-dependent MAPK phosphorylation, transcriptional induction, mating morphogenesis, and gradient tracking. These include both single-cell and population-based assays of activity. We describe several technical advances, provide example data for benchmark mutants, highlight important differences between newer and established methodologies, and compare the advantages and disadvantages of each as applied to the yeast model. Quantitative measurements of pathway activity have been used to develop mathematical models and reveal new regulatory mechanisms in yeast. It is our expectation that experimental and computational approaches developed in yeast may eventually be adapted to human systems biology and pharmacology.
Keywords: Saccharomyces cerevisiae; signal transduction; systems biology.
© 2019 John Wiley & Sons, Ltd.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Figures










References
-
- Apanovitch DM, Iiri T, Karasawa T, Bourne HR, & Dohlman HG (1998). Second site suppressor mutations of a GTPase-deficient G-protein alpha-subunit. Selective inhibition of Gbeta gamma-mediated signaling. The Journal of Biological Chemistry, 273(44), 28597–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
