Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6)
- PMID: 31023318
- PMCID: PMC6485075
- DOI: 10.1186/s12885-019-5545-0
Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6)
Abstract
Background: Upfront cytoreductive surgery with HIPEC (CRS-HIPEC) is the standard treatment for isolated resectable colorectal peritoneal metastases (PM) in the Netherlands. This study investigates whether addition of perioperative systemic therapy to CRS-HIPEC improves oncological outcomes.
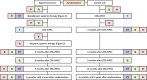
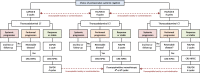
Methods: This open-label, parallel-group, phase II-III, randomised, superiority study is performed in nine Dutch tertiary referral centres. Eligible patients are adults who have a good performance status, histologically or cytologically proven resectable PM of a colorectal adenocarcinoma, no systemic colorectal metastases, no systemic therapy for colorectal cancer within six months prior to enrolment, and no previous CRS-HIPEC. Eligible patients are randomised (1:1) to perioperative systemic therapy and CRS-HIPEC (experimental arm) or upfront CRS-HIPEC alone (control arm) by using central randomisation software with minimisation stratified by a peritoneal cancer index of 0-10 or 11-20, metachronous or synchronous PM, previous systemic therapy for colorectal cancer, and HIPEC with oxaliplatin or mitomycin C. At the treating physician's discretion, perioperative systemic therapy consists of either four 3-weekly neoadjuvant and adjuvant cycles of capecitabine with oxaliplatin (CAPOX), six 2-weekly neoadjuvant and adjuvant cycles of 5-fluorouracil/leucovorin with oxaliplatin (FOLFOX), or six 2-weekly neoadjuvant cycles of 5-fluorouracil/leucovorin with irinotecan (FOLFIRI) followed by four 3-weekly (capecitabine) or six 2-weekly (5-fluorouracil/leucovorin) adjuvant cycles of fluoropyrimidine monotherapy. Bevacizumab is added to the first three (CAPOX) or four (FOLFOX/FOLFIRI) neoadjuvant cycles. The first 80 patients are enrolled in a phase II study to explore the feasibility of accrual and the feasibility, safety, and tolerance of perioperative systemic therapy. If predefined criteria of feasibility and safety are met, the study continues as a phase III study with 3-year overall survival as primary endpoint. A total of 358 patients is needed to detect the hypothesised 15% increase in 3-year overall survival (control arm 50%; experimental arm 65%). Secondary endpoints are surgical characteristics, major postoperative morbidity, progression-free survival, disease-free survival, health-related quality of life, costs, major systemic therapy related toxicity, and objective radiological and histopathological response rates.
Discussion: This is the first randomised study that prospectively compares oncological outcomes of perioperative systemic therapy and CRS-HIPEC with upfront CRS-HIPEC alone for isolated resectable colorectal PM.
Trial registration: Clinicaltrials.gov/ NCT02758951 , NTR/ NTR6301 , ISRCTN/ ISRCTN15977568 , EudraCT/ 2016-001865-99 .
Keywords: Adjuvant chemotherapy; Bevacizumab; Colorectal neoplasms; Cytoreduction surgical procedures; Hyperthermia, induced; Mortality; Neoadjuvant therapy; Peritoneal neoplasms; Progression-free survival; Randomized controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by a central ethics committee (Medical Research Ethics Committees United, Nieuwegein, Netherlands, R16.056), the Dutch Competent Authority (Centrale Commissie Mensgebonden Onderzoek, The Hague, Netherlands, NL57644.100.16), and the institutional review boards of all study centres. Written informed consent is obtained from all patients participating in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Perioperative Systemic Therapy vs Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Alone for Resectable Colorectal Peritoneal Metastases: A Phase 2 Randomized Clinical Trial.JAMA Surg. 2021 Aug 1;156(8):710-720. doi: 10.1001/jamasurg.2021.1642. JAMA Surg. 2021. PMID: 34009291 Free PMC article. Clinical Trial.
-
Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial.Lancet Oncol. 2021 Feb;22(2):256-266. doi: 10.1016/S1470-2045(20)30599-4. Epub 2021 Jan 18. Lancet Oncol. 2021. PMID: 33476595 Clinical Trial.
-
Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study.Lancet Oncol. 2020 Sep;21(9):1147-1154. doi: 10.1016/S1470-2045(20)30322-3. Epub 2020 Jul 24. Lancet Oncol. 2020. PMID: 32717180 Clinical Trial.
-
Systematic review of published literature on oxaliplatin and mitomycin C as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer.Crit Rev Oncol Hematol. 2019 Oct;142:119-129. doi: 10.1016/j.critrevonc.2019.06.014. Epub 2019 Jul 9. Crit Rev Oncol Hematol. 2019. PMID: 31400583
-
[Surgical treatment of peritoneal metastases of colorectal cancer].Chirurg. 2018 Sep;89(9):663-668. doi: 10.1007/s00104-018-0620-7. Chirurg. 2018. PMID: 29589077 Review. German.
Cited by
-
Hyperthermic intraperitoneal chemotherapy and colorectal cancer: From physiology to surgery.World J Clin Cases. 2022 Oct 26;10(30):10852-10861. doi: 10.12998/wjcc.v10.i30.10852. World J Clin Cases. 2022. PMID: 36338235 Free PMC article. Review.
-
Perioperative Systemic Therapy Versus Cytoreductive Surgery and HIPEC Alone for Resectable Colorectal Peritoneal Metastases: Patient-Reported Outcomes of a Randomized Phase II Trial.Ann Surg Oncol. 2023 May;30(5):2678-2688. doi: 10.1245/s10434-023-13116-z. Epub 2023 Feb 8. Ann Surg Oncol. 2023. PMID: 36754943 Free PMC article. Clinical Trial.
-
Impact of Neoadjuvant Chemotherapy on the Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Multi-Institutional Retrospective Review.J Clin Med. 2020 Mar 10;9(3):748. doi: 10.3390/jcm9030748. J Clin Med. 2020. PMID: 32164300 Free PMC article.
-
Anatomical Targeting of Anticancer Drugs to Solid Tumors Using Specific Administration Routes: Review.Pharmaceutics. 2023 Jun 6;15(6):1664. doi: 10.3390/pharmaceutics15061664. Pharmaceutics. 2023. PMID: 37376112 Free PMC article. Review.
-
Detection of tumor-derived cell-free DNA from colorectal cancer peritoneal metastases in plasma and peritoneal fluid.J Pathol Clin Res. 2021 May;7(3):203-208. doi: 10.1002/cjp2.207. Epub 2021 Feb 26. J Pathol Clin Res. 2021. PMID: 33635598 Free PMC article.
References
-
- van Gestel YR, Thomassen I, Lemmens VE, Pruijt JF, van Herk-Sukel MP, Rutten HJ, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. 2014;40:693–699. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

