ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway
- PMID: 31023337
- PMCID: PMC6482513
- DOI: 10.1186/s13046-019-1156-5
ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway
Abstract
Background: Breast cancer angiogenesis is key for metastasis and predicts a poor prognosis. Angiotensin-converting enzyme 2 (ACE2), as a member of the renin-angiotensin system (RAS), was reported to restrain the progression of hepatocellular carcinoma (HCC) and non-small cell lung cancer (NSCLC) through inhibiting angiogenesis. However, the relationship between ACE2 and breast cancer angiogenesis remains unclear.
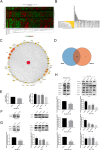
Methods: The prognosis and relative gene selection were analysed using the GEPIA, GEO, TCGA and STRING databases. ACE2 expression in breast cancer tissue was estimated by reverse transcription-quantitative polymerase chain reaction (qPCR). Breast cancer cell migration, proliferation and angiogenesis were assessed by Transwell migration, proliferation, tube formation, and wound healing assays. The expression of vascular endothelial growth factor A (VEGFa) was detected by qPCR and Western blotting. The phosphorylation of vascular endothelial growth factor receptor 2 (VEGFR2), mitogen-activated protein kinase 1/2 (MEK1/2), and extracellular signal-regulated protein kinase 1/2 (ERK1/2) was examined by Western blotting. Breast cancer metastasis and angiogenesis in vivo were measured using a zebrafish model.
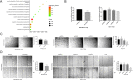
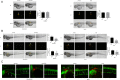
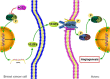
Results: ACE2 was downregulated in breast cancer patients. Patients with higher ACE2 expression had longer relapse-free survival (RFS). In vitro, ACE2 inhibited breast cancer migration. Meanwhile, ACE2 in breast cancer cells inhibited human umbilical vascular endothelial cell (HUVEC) proliferation, tube formation and migration. In the zebrafish model, ACE2 inhibited breast cancer cell metastasis, as demonstrated by analyses of the number of disseminated foci and the metastatic distance. Neo-angiogenesis was also decreased by ACE2. ACE2 downregulated the expression of VEGFa in breast cancer cells. Furthermore, ACE2 in breast cancer cells inactivated the phosphorylation of VEGFR2, MEK1/2, and ERK1/2 in HUVECs.
Conclusions: Our findings suggest that ACE2, as a potential resister to breast cancer, might inhibit breast cancer angiogenesis through the VEGFa/VEGFR2/ERK pathway.
Trial registration: Retrospectively registered.
Keywords: ACE2; Angiogenesis; Breast cancer; ERK; VEGFR2; VEGFa.
Conflict of interest statement
Ethics approval and consent to participate
Approval and consent obtained for the use of human tissue were obtained from the Institutional Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University.
Approval for all the zebrafish experiments was obtained from Sun Yat-sen University Animal Care and Use Committee of the Zebrafish Model Animal Facility, Institute of Clinical and Translational Research, Sun Yat-sen University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

