Is resistant hypertension an independent predictor of all-cause mortality in individuals with type 2 diabetes? A prospective cohort study
- PMID: 31023377
- PMCID: PMC6482506
- DOI: 10.1186/s12916-019-1313-x
Is resistant hypertension an independent predictor of all-cause mortality in individuals with type 2 diabetes? A prospective cohort study
Abstract
Background: Resistant hypertension is independently associated with an increased risk of death in the general hypertensive population. We assessed whether resistant hypertension is an independent predictor of all-cause mortality in individuals with type 2 diabetes from the Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicentre Study.
Methods: On 31 October 2015, vital status information was retrieved for 15,656 of the 15,773 participants enrolled in 2006-2008. Based on baseline blood pressure (BP) values and treatment, participants were categorized as normotensive, untreated hypertensive, controlled hypertensive (i.e., on-target with < 3 drugs), uncontrolled hypertensive (i.e., not on-target with 1-2 drugs), or resistant hypertensive (i.e., uncontrolled with > 3 drugs or controlled with > 4 drugs). Kaplan-Meier and Cox proportional hazards regression analyses were used to assess the association with all-cause mortality.
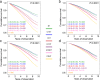
Results: Using the 130/80 mmHg targets for categorization, crude mortality rates and Kaplan-Meier estimates were highest among resistant hypertension participants, especially those with controlled resistant hypertension. As compared with resistant hypertension, risk for all-cause mortality was significantly lower for all the other groups, including individuals with controlled hypertension (hazard ratio 0.81 [95% confidence interval 0.74-0.89], P < 0.0001), but became progressively similar between resistant and controlled hypertension after adjustment for cardiovascular risk factors and complications/comorbidities. Also when compared with controlled resistant hypertension, mortality risk was significantly lower for all the other groups, including controlled hypertension, even after adjusting for cardiovascular risk factors (0.77 [0.63-0.95], P = 0.012), but not for complications/comorbidities (0.88 [0.72-1.08], P = 0.216). BP was well below target in the controlled hypertensive groups (resistant and non-resistant) and values < 120/70 mmHg were associated with an increased mortality risk. Results changed only partly when using the 140/90 mmHg targets for categorization.
Conclusions: In the RIACE cohort, at variance with the general hypertensive population, resistant hypertension did not predict death beyond target organ damage. Our findings may be explained by the high mortality risk conferred by type 2 diabetes and the low BP values observed in controlled hypertensive patients, which may mask risk associated with resistant hypertension. Less stringent BP goals may be preferable in high-risk patients with type 2 diabetes.
Trial registration: ClinicalTrials.gov, NCT00715481 , retrospectively registered 15 July, 2008.
Keywords: All-cause mortality; Cardiovascular disease; Chronic kidney disease; Resistant hypertension; Type 2 diabetes.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. The research protocol was approved by the ethics committee of the coordinating centre (Sant’Andrea Hospital, Rome Italy) on 25 September, 2006 (n. 43/2006) and subsequently by the ethics committee of each participating centre. Participants provided an informed consent.
Consent for publication
Not applicable.
Competing interests
AS: consulting fees from Astra-Zeneca, Boehringer–Ingelheim, Eli Lilly; lecture fees from Boehringer–Ingelheim, Eli Lilly, Sanofi-Aventis; grant support from Astra-Zeneca. GPe: consulting fees from Astra-Zeneca, Boehringer–Ingelheim, Eli Lilly; lecture fees from Astra-Zeneca, Boehringer–Ingelheim, Eli Lilly, Merck-Sharp&Dohme, Novo Nordisk. EO: consulting fees from Boehringer–Ingelheim, Eli Lilly, Novo Nordisk, Sanofi-Aventis; lecture fees from Abbot, Astra-Zeneca, Eli Lilly, Lifescan, Sanofi-Aventis, Takeda. EB: consulting fees from Abbott, Astra-Zeneca, Boehringer–Ingelheim, Bruno Farmaceutici, Eli Lilly; lecture fees from Bristol-Myers Squibb, Eli Lilly, Janssen, Merck-Sharp&Dohme, Novartis, Novo Nordisk, Roche, Sanofi-Aventis, Servier, Takeda; grant support from Astra-Zeneca, Genzyme, Menarini Diagnostics, Novo Nordisk, Roche, Takeda. CF: none reported. RT: consulting fees from Boehringer–Ingelheim, Sanofi-Aventis; lecture fees from Astra-Zeneca, Boehringer–Ingelheim, Eli Lilly, Janssen, Medtronic, Novartis, Novo Nordisk, Sanofi-Aventis; grant support from Astra-Zeneca, Boehringer–Ingelheim, Eli Lilly, Janssen, Novo Nordisk, Sanofi-Aventis. MV: none reported. FC: lecture fees from Astra-Zeneca, Boehringer–Ingelheim, Merck-Sharp&Dohme, Sanofi-Aventis, Takeda. OL: consulting fees from Astra-Zeneca, Boehringer–Ingelheim; lecture fees from Astra-Zeneca, Eli Lilly, Merck-Sharp&Dohme, Sigma-Tau, Sanofi-Aventis, Takeda; grant support from Astra-Zeneca. MB: lecture fees from Abbot, Astra-Zeneca, Mundi Pharma, Novo Nordisk, Sanofi-Aventis; grant support from Sanofi-Aventis. AN: consulting fees from Eli Lilly, Novo Nordisk; lecture fees from Eli Lilly, Novo Nordisk; grant support from AlfaSigma, Artsana, Astra-Zeneca, Eli Lilly, Novo Nordisk, Sanofi-Aventis. GPu: lecture fees from Astra-Zeneca, Eli Lilly, Sigma-Tau, Takeda.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Pagidipati NJ, Navar AM, Pieper KS, Green JB, Bethel MA, Armstrong PW, et al. Secondary prevention of cardiovascular disease in patients with type 2 diabetes mellitus: international insights from the TECOS Trial (Trial Evaluating Cardiovascular Outcomes With Sitagliptin) Circulation. 2017;136:1193–1203. doi: 10.1161/CIRCULATIONAHA.117.027252. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

