Aberrant Clonal Hematopoiesis following Lentiviral Vector Transduction of HSPCs in a Rhesus Macaque
- PMID: 31023523
- PMCID: PMC6554657
- DOI: 10.1016/j.ymthe.2019.04.003
Aberrant Clonal Hematopoiesis following Lentiviral Vector Transduction of HSPCs in a Rhesus Macaque
Abstract
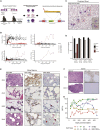
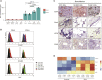
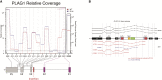
Lentiviral vectors (LVs) are used for delivery of genes into hematopoietic stem and progenitor cells (HSPCs) in clinical trials worldwide. LVs, in contrast to retroviral vectors, are not associated with insertion site-associated malignant clonal expansions and, thus, are considered safer. Here, however, we present a case of markedly abnormal dysplastic clonal hematopoiesis affecting the erythroid, myeloid, and megakaryocytic lineages in a rhesus macaque transplanted with HSPCs that were transduced with a LV containing a strong retroviral murine stem cell virus (MSCV) constitutive promoter-enhancer in the LTR. Nine insertions were mapped in the abnormal clone, resulting in overexpression and aberrant splicing of several genes of interest, including the cytokine stem cell factor and the transcription factor PLAG1. This case represents the first clear link between lentiviral insertion-induced clonal expansion and a clinically abnormal transformed phenotype following transduction of normal primate or human HSPCs, which is concerning, and suggests that strong constitutive promoters should not be included in LVs.
Keywords: gene therapy; genotoxicity; lentiviral vector; non-human primate.
Published by Elsevier Inc.
Figures


Comment in
-
Gene Transfer to HSCs: Finding the Leukemia in Murine Leukemia Viruses.Mol Ther. 2019 Jun 5;27(6):1072-1073. doi: 10.1016/j.ymthe.2019.05.003. Epub 2019 May 17. Mol Ther. 2019. PMID: 31109833 Free PMC article. No abstract available.
References
-
- Naldini L., Trono D., Verma I.M. Lentiviral vectors, two decades later. Science. 2016;353:1101–1102. - PubMed
-
- Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. - PubMed
-
- Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Veres G., Schmidt M., Kutschera I., Vidaud M., Abel U., Dal-Cortivo L., Caccavelli L. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

