Interaction of the late endo-lysosomal lipid PI(3,5)P2 with the Vph1 isoform of yeast V-ATPase increases its activity and cellular stress tolerance
- PMID: 31023825
- PMCID: PMC6556579
- DOI: 10.1074/jbc.RA119.008552
Interaction of the late endo-lysosomal lipid PI(3,5)P2 with the Vph1 isoform of yeast V-ATPase increases its activity and cellular stress tolerance
Abstract
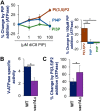
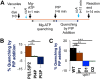
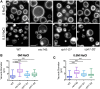
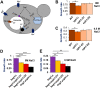
The low-level endo-lysosomal signaling lipid, phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2), is required for full assembly and activity of vacuolar H+-ATPases (V-ATPases) containing the vacuolar a-subunit isoform Vph1 in yeast. The cytosolic N-terminal domain of Vph1 is also recruited to membranes in vivo in a PI(3,5)P2-dependent manner, but it is not known if its interaction with PI(3,5)P2 is direct. Here, using biochemical characterization of isolated yeast vacuolar vesicles, we demonstrate that addition of exogenous short-chain PI(3,5)P2 to Vph1-containing vacuolar vesicles activates V-ATPase activity and proton pumping. Modeling of the cytosolic N-terminal domain of Vph1 identified two membrane-oriented sequences that contain clustered basic amino acids. Substitutions in one of these sequences (231KTREYKHK) abolished the PI(3,5)P2-dependent activation of V-ATPase without affecting basal V-ATPase activity. We also observed that vph1 mutants lacking PI(3,5)P2 activation have enlarged vacuoles relative to those in WT cells. These mutants exhibit a significant synthetic growth defect when combined with deletion of Hog1, a kinase important for signaling the transcriptional response to osmotic stress. The results suggest that PI(3,5)P2 interacts directly with Vph1, and that this interaction both activates V-ATPase activity and protects cells from stress.
Keywords: Saccharomyces cerevisiae; acidification; lysosome; osmoregulation; osmotic stress; phosphatidylinositol 3,5-bisphosphate; phosphatidylinositol signaling; proton pump; vacuolar ATPase; vacuole.
© 2019 Banerjee et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

