Sulfenamide and Sulfonamide Derivatives of Metformin - A New Option to Improve Endothelial Function and Plasma Haemostasis
- PMID: 31024058
- PMCID: PMC6484023
- DOI: 10.1038/s41598-019-43083-z
Sulfenamide and Sulfonamide Derivatives of Metformin - A New Option to Improve Endothelial Function and Plasma Haemostasis
Abstract
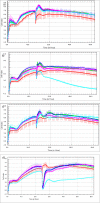
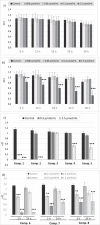
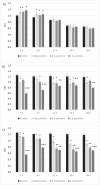
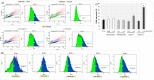
Type 2 diabetes mellitus (T2DM) is a multi-factorial disease which can cause multiple organ dysfunction, including that of the vascular endothelium. The aim of the present study was to evaluate the effects of metformin, and its sulfenamide and sulfonamide derivatives (compounds 1-8) on the selected markers of endothelial function and blood coagulation. The integrity of endothelial cells(ECs) was examined using the real-time cell electric impedance system. Tissue Factor(TF) production, the release of von Willebrand Factor (vWF) and tissue plasminogen activator(t-PA) from ECs were determined using immunoenzymatic assays, while the process of platelet thrombus formation using the Total Thrombus-Formation Analysis System. Sulfenamide with n-butyl alkyl chain(3) does not interfere with ECs integrity, and viability (nCI(24h) = 1.03 ± 0.03 vs. 1.06 ± 0.11 for control), but possesses anticoagulation properties manifested by prolonged platelet-dependent thrombus formation (Occlusion Time 370.3 ± 77.0 s vs. 286.7 ± 65.5 s for control) in semi-physiological conditions. Both p- and o-nitro-benzenesulfonamides (compounds7,8) exhibit anti-coagulant properties demonstrated by decreased vWF release and prolonged parameters of platelet thrombus formation and total blood thrombogenicity. In conclusion, chemical modification of metformin scaffold into sulfenamides or sulfonamides might be regarded as a good starting point for the design and synthesis of novel biguanide-based compounds with anticoagulant properties and valuable features regarding endothelial function.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Kooy, A., Zeeuw, D. D. E., Stehouwer, C. D. A. & Gansevoort, R. T. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. 1–14 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

