Metabolic Pathways and Potencies of New Fentanyl Analogs
- PMID: 31024296
- PMCID: PMC6461066
- DOI: 10.3389/fphar.2019.00238
Metabolic Pathways and Potencies of New Fentanyl Analogs
Abstract
Up to now, little is known about the metabolic pathways of new fentanyl analogs that have recently emerged on the drug markets worldwide with high potential for producing addiction and severe adverse effects including coma and death. For some of the compounds, limited information on the metabolism has been published, however, for others so far no information is available. Considering the well characterized metabolism of the pharmaceutically used opioid fentanyl and the so far available data, the metabolism of the new fentanyl analogs can be anticipated to generally involve reactions like hydrolysis, hydroxylation (and further oxidation steps), N- and O-dealkylation and O-methylation. Furthermore, phase II metabolic reactions can be expected comprising glucuronide or sulfate conjugate formation. When analyzing blood and urine samples of acute intoxication cases or fatalities, the presence of metabolites can be crucial for confirmation of the uptake of such compounds and further interpretation. Here we present a review on the metabolic profiles of new fentanyl analogs responsible for a growing number of severe and fatal intoxications in the United States, Europe, Canada, Australia, and Japan in the last years, as assessed by a systematic search of the scientific literature and official reports.
Keywords: fentanyl analogs; fentanyl biotransformations; in vivo and in vitro metabolism; metabolic profile; novel synthetic opioids; receptor binding affinity; toxicity.
Figures
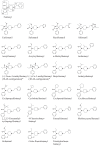
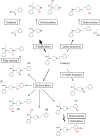
References
-
- Andreasen M. F., Hardlei T. F., Rosendal I., Thomsen A. H., Johannsen M., Saedder E. (2017). “A fatal poisoning involving 2-fluorofentanyl,” in Proceedings of the 55th SOFT-TIAFT 2017 Annual Meeting, Boca Raton, FL.
-
- Åstrand A., Töreskog A., Watanabe S., Kronstrand R., Gréen H., Vikingsson S. (2018). Correlations between metabolism and structural elements of the alicyclic fentanyl analogs cyclopropyl fentanyl, cyclobutyl fentanyl, cyclopentyl fentanyl, cyclohexyl fentanyl and 2,2,3,3-tetramethylcyclopropyl fentanyl studied by human hepatocytes and LC-QTOF-MS. Arch. Toxicol. 93 95–106. 10.1007/s00204-018-2330-9 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

