Spatiotemporal Modeling of the Key Migratory Events During the Initiation of Adaptive Immunity
- PMID: 31024523
- PMCID: PMC6460458
- DOI: 10.3389/fimmu.2019.00598
Spatiotemporal Modeling of the Key Migratory Events During the Initiation of Adaptive Immunity
Abstract
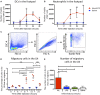
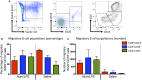
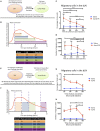
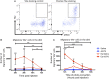
Initiation of adaptive immunity involves distinct migratory cell populations coming together in a highly dynamic and spatially organized process. However, we lack a detailed spatiotemporal map of these events due to our inability to track the fate of cells between anatomically distinct locations or functionally identify cell populations as migratory. We used photo-convertible transgenic mice (Kaede) to spatiotemporally track the fate and composition of the cell populations that leave the site of priming and enter the draining lymph node to initiate immunity. We show that following skin priming, the lymph node migratory population is principally composed of cells recruited to the site of priming, with a minor contribution from tissue resident cells. In combination with the YAe/Eα system, we also show that the majority of cells presenting antigen are CD103+CD11b+ dendritic cells that were recruited to the site of priming during the inflammatory response. This population has previously only been described in relation to mucosal tissues. Comprehensive phenotypic profiling of the cells migrating from the skin to the draining lymph node by mass cytometry revealed that in addition to dendritic cells, the migratory population also included CD4+ and CD8+ T cells, B cells, and neutrophils. Taking our complex spatiotemporal data set, we then generated a model of cell migration that quantifies and describes the dynamics of arrival, departure, and residence times of cells at the site of priming and in the draining lymph node throughout the time-course of the initiation of adaptive immunity. In addition, we have identified the mean migration time of migratory dendritic cells as they travel from the site of priming to the draining lymph node. These findings represent an unprecedented, detailed and quantitative map of cell dynamics and phenotypes during immunization, identifying where, when and which cells to target for immunomodulation in autoimmunity and vaccination strategies.
Keywords: adaptive immunity; cell migration; cell tracking; dendritic cells; innate immunity.
Figures


Comment in
-
Commentary: Spatiotemporal Modeling of the Key Migratory Events During the Initiation of Adaptive Immunity.Front Immunol. 2019 Sep 24;10:2311. doi: 10.3389/fimmu.2019.02311. eCollection 2019. Front Immunol. 2019. PMID: 31681263 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

