Kctd9 Deficiency Impairs Natural Killer Cell Development and Effector Function
- PMID: 31024568
- PMCID: PMC6467973
- DOI: 10.3389/fimmu.2019.00744
Kctd9 Deficiency Impairs Natural Killer Cell Development and Effector Function
Abstract
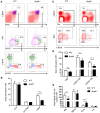
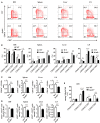
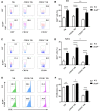
We previously showed that potassium channel tetramerization domain containing 9 (KCTD9) is aberrantly expressed in natural killer (NK) cells in patients with hepatitis B virus-associated acute-on-chronic liver failure and mice with experimental fulminant hepatitis. However, the mechanism underlying the regulation of NK cell function and fulminant hepatitis progression by KCTD9 is unknown. Here, we investigated the role of Kctd9 in regulation of early development, maturation, and function of NK cells using Kctd9-knockout mice. Compared to wild-type mice, Kctd9-deficient mice exhibited impaired NK cell lineage commitment, as evidenced by selective reduction in the refined NK progenitors, and incomplete NK cell maturation, as manifested by a higher proportion of CD11b- NK cells and a lower percentage of CD11b+ NK cells with high proliferative potential. Moreover, Kctd9-depleted NK cells displayed insufficient IFN-γ production, degranulation, and granzyme B production in response to cytokine stimulation, and attenuated cytotoxicity to tumor cells in vitro. The defect in NK cells was further supported by ameliorated liver damage and improved survival in Kctd9-deficient mice following murine hepatitis virus strain-3 (MHV-3) infection, which otherwise leads to immune-mediated fulminant hepatitis, a phenotype homologous to that caused by NK cell depletion in wild-type mice. Further investigation to identify the underlying mechanism revealed that Kctd9 deficiency hindered the expression of transcription factors, including Ets1, Nfil3, Eomes, and Id2 in NK cells. Collectively, our data reveal that Kctd9 acts as a novel regulator for NK cell commitment, maturation, and effector function.
Keywords: Kctd9; NK cells; development; fulminant hepatitis; liver damage.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

