Characterization of the iPSC-derived conditioned medium that promotes the growth of bovine corneal endothelial cells
- PMID: 31024764
- PMCID: PMC6474332
- DOI: 10.7717/peerj.6734
Characterization of the iPSC-derived conditioned medium that promotes the growth of bovine corneal endothelial cells
Abstract
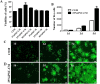
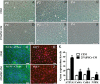
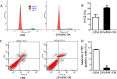
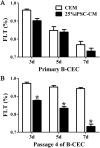
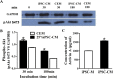
Corneal endothelial cells (CECs) maintain corneal transparency and visual acuity. However, the limited proliferative capability of these cells in vitro has prompted researchers to find efficient culturing techniques for them. The aim of our study was to evaluate the use of conditioned medium (CM) obtained from induced pluripotent stem cells (iPSCs) as a source for the effective proliferation of bovine CECs (B-CECs). In our study, the proliferative ability of B-CECs was moderately enhanced when the cells were grown in 25% iPSC conditioned medium (iPSC-CM). Additionally, hexagonal cell morphology was maintained until passage 4, as opposed to the irregular and enlarged shape observed in control corneal endothelial medium (CEM). B-CECs in both the 25% iPSC-CM and CEM groups expressed and Na+-K+-ATPase. The gene expression levels of NIFK, Na+-K+-ATPase, Col4A and Col8A and the percentage of cells entering S and G2 phases were higher in the iPSC-CM group. The number of apoptotic cells also decreased in the iPSC-CM group. In comparison to the control cultures, iPSC-CM facilitated cell migration, and these cells showed better barrier functions after several passages. The mechanism of cell proliferation mediated by iPSC-CM was also investigated, and phosphorylation of Akt was observed in B-CECs after exposure to iPSC-CM and showed sustained phosphorylation induced for up to 180 min in iPSC-CM. Our findings indicate that iPSC-CM may employ PI3-kinase signaling in regulating cell cycle progression, which can lead to enhanced cellular proliferation. Effective component analysis of the CM showed that in the iPSC-CM group, the expression of activin-A was significantly increased. If activin-A is added as a supplement, it could help to maintain the morphology of the cells, similar to that of CM. Hence, we conclude that activin-A is one of the effective components of CM in promoting cell proliferation and maintaining cell morphology.
Keywords: Conditioned medium; Corneal endothelial cells (CECs); Induction pluripotent stem cells.
Conflict of interest statement
Jiansu Chen and Zekai Cui are employed by Aier Eye Institute, Changsha, Hunan, China. The authors declare that they have no competing interests.
Figures


References
-
- Bednarz J, Teifel M, Friedl P, Engelmann K. Immortalization of human corneal endothelial cells using electroporation protocol optimized for human corneal endothelial and human retinal pigment epithelial cells. Acta Ophthalmologica Scandinavica. 2000;78(2):130–136. doi: 10.1034/j.1600-0420.2000.078002130.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

