Macrophages Infected by a Pathogen and a Non-pathogen Spotted Fever Group Rickettsia Reveal Differential Reprogramming Signatures Early in Infection
- PMID: 31024862
- PMCID: PMC6467950
- DOI: 10.3389/fcimb.2019.00097
Macrophages Infected by a Pathogen and a Non-pathogen Spotted Fever Group Rickettsia Reveal Differential Reprogramming Signatures Early in Infection
Abstract
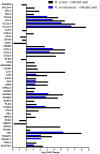
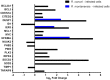
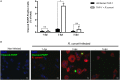
Despite their high degree of genomic similarity, different spotted fever group (SFG) Rickettsia are often associated with very different clinical presentations. For example, Rickettsia conorii causes Mediterranean spotted fever, a life-threatening disease for humans, whereas Rickettsia montanensis is associated with limited or no pathogenicity to humans. However, the molecular basis responsible for the different pathogenicity attributes are still not understood. Although killing microbes is a critical function of macrophages, the ability to survive and/or proliferate within phagocytic cells seems to be a phenotypic feature of several intracellular pathogens. We have previously shown that R. conorii and R. montanensis exhibit different intracellular fates within macrophage-like cells. By evaluating early macrophage responses upon insult with each of these rickettsial species, herein we demonstrate that infection with R. conorii results in a profound reprogramming of host gene expression profiles. Transcriptional programs generated upon infection with this pathogenic bacteria point toward a sophisticated ability to evade innate immune signals, by modulating the expression of several anti-inflammatory molecules. Moreover, R. conorii induce the expression of several pro-survival genes, which may result in the ability to prolong host cell survival, thus protecting its replicative niche. Remarkably, R. conorii-infection promoted a robust modulation of different transcription factors, suggesting that an early manipulation of the host gene expression machinery may be key to R. conorii proliferation in THP-1 macrophages. This work provides new insights into the early molecular processes hijacked by a pathogenic SFG Rickettsia to establish a replicative niche in macrophages, opening several avenues of research in host-rickettsiae interactions.
Keywords: Rickettsia conorii; Rickettsia montanensis; host-pathogen interactions; macrophages; spotted fever group Rickettsia; transcriptional profiling.
Figures





References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

