Plasma from Volunteers Breathing Helium Reduces Hypoxia-Induced Cell Damage in Human Endothelial Cells-Mechanisms of Remote Protection Against Hypoxia by Helium
- PMID: 31025141
- PMCID: PMC6538579
- DOI: 10.1007/s10557-019-06880-2
Plasma from Volunteers Breathing Helium Reduces Hypoxia-Induced Cell Damage in Human Endothelial Cells-Mechanisms of Remote Protection Against Hypoxia by Helium
Abstract
Purpose: Remote ischemic preconditioning protects peripheral organs against prolonged ischemia/reperfusion injury via circulating protective factors. Preconditioning with helium protected healthy volunteers against postischemic endothelial dysfunction. We investigated whether plasma from helium-treated volunteers can protect human umbilical vein endothelial cells (HUVECs) against hypoxia in vitro through release of circulating of factors.
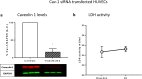
Methods: Healthy male volunteers inhaled heliox (79% helium, 21% oxygen) or air for 30 min. Plasma was collected at baseline, directly after inhalation, 6 h and 24 h after start of the experiment. HUVECs were incubated with either 5% or 10% of the plasma for 1 or 2 h and subjected to enzymatically induced hypoxia. Cell damage was measured by LDH content. Furthermore, caveolin 1 (Cav-1), hypoxia-inducible factor (HIF1α), extracellular signal-regulated kinase (ERK)1/2, signal transducer and activator of transcription (STAT3) and endothelial nitric oxide synthase (eNOS) were determined.
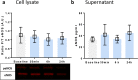
Results: Prehypoxic exposure to 10% plasma obtained 6 h after helium inhalation decreased hypoxia-induced cell damage in HUVEC. Cav-1 knockdown in HUVEC abolished this effect.
Conclusions: Plasma of healthy volunteers breathing helium protects HUVEC against hypoxic cell damage, possibly involving circulating Cav-1.
Keywords: Caveolin-1; Endothelial conditioning; Helium; Ischemic preconditioning; Remote.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


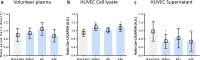

References
-
- Pagel PS, Krolikowski JG, Shim YH, Venkatapuram S, Kersten JR, Weihrauch D, Warltier DC, Pratt PF., Jr Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth Analg. 2007;105(3):562–569. doi: 10.1213/01.ane.0000278083.31991.36. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

