A Structured Preapproval and Postapproval Comparative Study Design Framework to Generate Valid and Transparent Real-World Evidence for Regulatory Decisions
- PMID: 31025311
- PMCID: PMC6771466
- DOI: 10.1002/cpt.1480
A Structured Preapproval and Postapproval Comparative Study Design Framework to Generate Valid and Transparent Real-World Evidence for Regulatory Decisions
Abstract
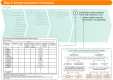
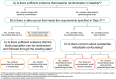
Real-world evidence provides important information about the effects of medicines in routine clinical practice. To engender trust that evidence generated for regulatory purposes is sufficiently valid, transparency in the reasoning that underlies study design decisions is critical. Building on existing guidance and frameworks, we developed the Structured Preapproval and Postapproval Comparative study design framework to generate valid and transparent real-world Evidence (SPACE) as a process for identifying design elements and minimal criteria for feasibility and validity concerns, and for documenting decisions. Starting with an articulated research question, we identify key components of the randomized controlled trial needed to maximize validity, and pragmatic choices are considered when required. A causal diagram is used to justify the variables identified for confounding control, and key decisions, assumptions, and evidence are captured in a structured way. In this way, SPACE may improve dialogue and build trust among healthcare providers, patients, regulators, and researchers.
© 2019 The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
N.M.G., R.F.R, and U.B.C. are full‐time employees and stock/shareholders of Pfizer.
Figures




References
-
- US Food and Drug Administration (FDA) . Framework for FDA's Real‐World Evidence Program. 1−37 <https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/RealWorldEvi...> (2018). Accessed April 15, 2019
-
- European Medicines Agency . Adaptive Pathways Workshop: Report on a Meeting with STAKEHOLDERS at EMA on 8 December 2016 (European Medicines Agency, London, England, 2017).
-
- Franklin, J.M. , Glynn, R.J. , Martin, D. & Schneeweiss, S. Evaluating the use of nonrandomized real‐world data analyses for regulatory decision making. Clin. Pharmacol. Ther. 105, 2019 (2019). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

