Advancements in magnetic resonance imaging-based biomarkers for muscular dystrophy
- PMID: 31026060
- PMCID: PMC8132307
- DOI: 10.1002/mus.26497
Advancements in magnetic resonance imaging-based biomarkers for muscular dystrophy
Abstract
Recent years have seen steady progress in the identification of genetic muscle diseases as well as efforts to develop treatment for these diseases. Consequently, sensitive and objective new methods are required to identify and monitor muscle pathology. Magnetic resonance imaging offers multiple potential biomarkers of disease severity in the muscular dystrophies. This Review uses a pathology-based approach to examine the ways in which MRI and spectroscopy have been used to study muscular dystrophies. Methods that have been used to quantitate intramuscular fat, edema, fiber orientation, metabolism, fibrosis, and vascular perfusion are examined, and this Review describes how MRI can help diagnose these conditions and improve upon existing muscle biomarkers by detecting small increments of disease-related change. Important challenges in the implementation of imaging biomarkers, such as standardization of protocols and validating imaging measurements with respect to clinical outcomes, are also described.
Keywords: magnetic resonance imaging; magnetic resonance spectroscopy; muscular dystrophy; outcome measures; quantitative imaging; skeletal muscle biomarkers.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
Disclosure of Conflicts of Interest:
The author does not have any conflicts of interest to disclose.
Figures

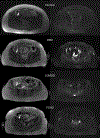


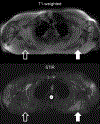

References
-
- Straub V, Murphy A, Udd B. 229th ENMC international workshop: Limb girdle muscular dystrophies - Nomenclature and reformed classification Naarden, the Netherlands, 17–19 March 2017. Neuromuscul Disord 2018;28:702–710. - PubMed
-
- Tordjman M, Dabaj I, Laforet P, Felter A, Ferreiro A, Biyoukar M, et al. Muscular MRI-based algorithm to differentiate inherited myopathies presenting with spinal rigidity. Eur Radiol 2018;28:5293–5303. - PubMed
-
- Mercuri E, Clements E, Offiah A, Pichiecchio A, Vasco G, Bianco F, et al. Muscle magnetic resonance imaging involvement in muscular dystrophies with rigidity of the spine. Ann Neurol 2010;67:201–208. - PubMed
-
- Barsotti S, Zampa V, Talarico R, Minichilli F, Ortori S, Iacopetti V, et al. Thigh magnetic resonance imaging for the evaluation of disease activity in patients with idiopathic inflammatory myopathies followed in a single center. Muscle Nerve 2016;54:666–672. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

