Vaccination with a chikungunya virus-like particle vaccine exacerbates disease in aged mice
- PMID: 31026260
- PMCID: PMC6485612
- DOI: 10.1371/journal.pntd.0007316
Vaccination with a chikungunya virus-like particle vaccine exacerbates disease in aged mice
Abstract
Introduction: Chikungunya virus (CHIKV) is a re-emerging pathogen responsible for causing outbreaks of febrile disease accompanied with debilitating joint pain. Symptoms typically persist for two weeks, but more severe and chronic chikungunya illnesses have been reported, especially in the elderly. Currently, there are no licensed vaccines or antivirals against CHIKV available. In this study, we combined a CHIK virus-like particle (VLP) vaccine with different adjuvants to enhance immunogenicity and protection in both, adult and aged mice.
Methods: CHIK VLP-based vaccines were tested in 6-8-week-old (adult) and 18-24-month-old (aged) female C57BL/6J mice. Formulations contained CHIK VLP alone or adjuvants: QuilA, R848, or Imject Alum. Mice were vaccinated three times via intramuscular injections. CHIKV-specific antibody responses were characterized by IgG subclass using ELISA, and by microneutralization assays. In addition, CHIKV infections were characterized in vaccinated and non-vaccinated adult mice and compared to aged mice.
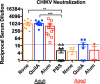
Results: In adult mice, CHIKV infection of the right hind foot induced significant swelling, which peaked by day 7 post-infection at approximately 170% of initial size. Viral titers peaked at 2.53 × 1010 CCID50/ml on day 2 post-infection. Mice vaccinated with CHIK VLP-based vaccines developed robust anti-CHIKV-specific IgG antibody responses that were capable of neutralizing CHIKV in vitro. CHIK VLP alone or CHIK plus QuilA administered by IM injections protected 100% of mice against CHIKV. In contrast, the antibody responses elicited by the VLP-based vaccines were attenuated in aged mice, with negligible neutralizing antibody titers detected. Unvaccinated, aged mice were resistant to CHIKV infection, while vaccination with CHIKV VLPs exacerbated disease.
Conclusions: Unadjuvanted CHIK VLP vaccination elicits immune responses that protect 100% of adult mice against CHIKV infection. However, an improved vaccine/adjuvant combination is still necessary to enhance the protective immunity against CHIKV in the aged.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



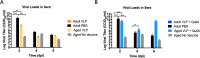

References
-
- CDC. Chikungunya Virus Geographic Distribution 2015 [updated May 12, 2016. Available from: http://www.cdc.gov/chikungunya/geo/index.html.
-
- WHO. Chikungunya fact sheet 2017 [updated April 2017; cited 2017 December 1, 2017]. Available from: http://www.who.int/mediacentre/factsheets/fs327/en/.
-
- Manica M, Guzzetta G, Poletti P, Filipponi F, Solimini A, Caputo B, et al. Transmission dynamics of the ongoing chikungunya outbreak in Central Italy: from coastal areas to the metropolitan city of Rome, summer 2017. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2017;22(44). 10.2807/1560-7917.ES.2017.22.44.17-00685 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

