Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications
- PMID: 31027272
- PMCID: PMC6523186
- DOI: 10.3390/polym11040745
Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications
Abstract
The field of polymeric nanoparticles is quickly expanding and playing a pivotal role in a wide spectrum of areas ranging from electronics, photonics, conducting materials, and sensors to medicine, pollution control, and environmental technology. Among the applications of polymers in medicine, gene therapy has emerged as one of the most advanced, with the capability to tackle disorders from the modern era. However, there are several barriers associated with the delivery of genes in the living system that need to be mitigated by polymer engineering. One of the most crucial challenges is the effectiveness of the delivery vehicle or vector. In last few decades, non-viral delivery systems have gained attention because of their low toxicity, potential for targeted delivery, long-term stability, lack of immunogenicity, and relatively low production cost. In 1987, Felgner et al. used the cationic lipid based non-viral gene delivery system for the very first time. This breakthrough opened the opportunity for other non-viral vectors, such as polymers. Cationic polymers have emerged as promising candidates for non-viral gene delivery systems because of their facile synthesis and flexible properties. These polymers can be conjugated with genetic material via electrostatic attraction at physiological pH, thereby facilitating gene delivery. Many factors influence the gene transfection efficiency of cationic polymers, including their structure, molecular weight, and surface charge. Outstanding representatives of polymers that have emerged over the last decade to be used in gene therapy are synthetic polymers such as poly(l-lysine), poly(l-ornithine), linear and branched polyethyleneimine, diethylaminoethyl-dextran, poly(amidoamine) dendrimers, and poly(dimethylaminoethyl methacrylate). Natural polymers, such as chitosan, dextran, gelatin, pullulan, and synthetic analogs, with sophisticated features like guanidinylated bio-reducible polymers were also explored. This review outlines the introduction of polymers in medicine, discusses the methods of polymer synthesis, addressing top down and bottom up techniques. Evaluation of functionalization strategies for therapeutic and formulation stability are also highlighted. The overview of the properties, challenges, and functionalization approaches and, finally, the applications of the polymeric delivery systems in gene therapy marks this review as a unique one-stop summary of developments in this field.
Keywords: biodistribution; blood circulation of polymeric nanoparticles; cellular internalization of the polymeric nanoparticles; colloidal stability of polymeric nanoparticles; cytotoxicity; green chemistry; top down and bottom up synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

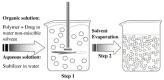
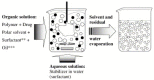
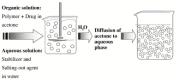
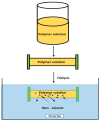
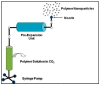
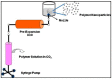
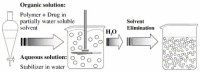
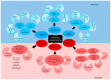
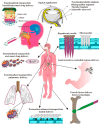

References
-
- Hosseinkhani H., Abedini F., Ou K.-L., Domb A. Polymers in gene therapy technology. Polym. Adv. Technol. 2015;26:198–211. doi: 10.1002/pat.3432. - DOI
-
- Alnylam Pharmaceuticals Inc. Alnylam Announces First-Ever FDA Approval of an RNAi Therapeutic, ONPATTRO™ (patisiran) for the Treatment of the Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis in Adults. [(accessed on 23 April 2019)]; Available online: http://investors.alnylam.com/news-releases/news-release-details/alnylam-....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

