Cell-Free Demineralized Bone Matrix for Mesenchymal Stem Cells Survival and Colonization
- PMID: 31027339
- PMCID: PMC6538993
- DOI: 10.3390/ma12091360
Cell-Free Demineralized Bone Matrix for Mesenchymal Stem Cells Survival and Colonization
Abstract
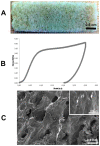
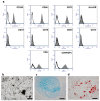
Decellularized bone matrix is receiving much attention as biological scaffolds and implantable biomaterials for bone tissue regeneration. Here, we evaluated the efficacy of a cell-free demineralized bone matrix on mesenchymal stem cells (MSCs) survival and differentiation in vitro. The seeding of human umbilical cord-derived MSCs (hUC-SCs) on decellularized bone matrices up to 14 days was exploited, assessing their capability of scaffold colonization and evaluating gene expression of bone markers. Light and Scanning Electron Microscopies were used. The obtained cell-free decalcified structures showed elastic moduli attributable to both topology and biochemical composition. Morphological observation evidenced an almost complete colonization of the scaffolds after 14 days of culture. Moreover, in hUC-SCs cultured on decalcified scaffolds, without the addition of any osteoinductive media, there was an upregulation of Collagen Type I (COL1) and osteonectin (ON) gene expression, especially on day 14. Modifications in the expression of genes engaged in stemness were also detected. In conclusion, the proposed decellularized bone matrix can induce the in vitro hUC-SCs differentiation and has the potential to be tested for in in vivo tissue regeneration.
Keywords: MSCs; decellularized bone matrix; gene expression; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rossi F., Santoro M., Perale G. Polymeric scaffolds as stem cell carriers in bone repair. J. Tissue Eng. Regen. Med. 2015;9:1093–1119. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

