Efficacy and safety of memantine in children with autism spectrum disorder: Results from three phase 2 multicenter studies
- PMID: 31027422
- PMCID: PMC6779018
- DOI: 10.1177/1362361318824103
Efficacy and safety of memantine in children with autism spectrum disorder: Results from three phase 2 multicenter studies
Abstract
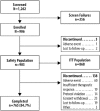
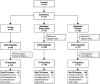
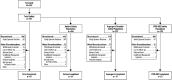
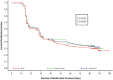
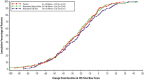
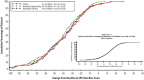
Three phase 2 trials were conducted to assess the efficacy and long-term safety of weight-based memantine extended release (ER) treatment in children with autism spectrum disorder. MEM-MD-91, a 50-week open-label trial, identified memantine extended-release treatment responders for enrollment into MEM-MD-68, a 12-week randomized, double-blind, placebo-controlled withdrawal trial. MEM-MD-69 was an open-label extension trial in which participants from MEM-MD-68, MEM-MD-91, and open-label trial MEM-MD-67 were treated ⩽48 weeks with memantine extended release. In MEM-MD-91, 517 (59.6%) participants were confirmed Social Responsiveness Scale responders at week 12; mean Social Responsiveness Scale total raw scores improved two to three times a minimal clinically important difference of 10 points. In MEM-MD-68, there was no difference between memantine and placebo on the primary efficacy parameter, the proportion of patients with a loss of therapeutic response (defined as ⩾10-point increase from baseline in Social Responsiveness Scale total raw score). MEM-MD-69 exploratory analyses revealed mean standard deviation improvement in Social Responsiveness Scale total raw score of 32.4 (26.4) from baseline of the first lead-in study. No new safety concerns were evident. While the a priori-defined efficacy results of the double-blind trial were not achieved, the considerable improvements in mean Social Responsiveness Scale scores from baseline in the open-label trials were presumed to be clinically important.
Keywords: Asperger’s disorder; Social Responsiveness Scale; autism spectrum disorders; clinical trial; medication; memantine; pervasive developmental disorder-not otherwise specified; randomized withdrawal; school-age children.
Figures

References
-
- Aman M. G., Findling R. L., Hardan A. Y., Hendren R. L., Melmed R. D., Kehinde-Nelson O., . . . Katz E. (2016). Safety and efficacy of memantine in children with autism: Randomized, placebo-controlled study and open-label extension. Journal of Child and Adolescent Psychopharmacology, 27, 403–412. - PMC - PubMed
-
- Aman M. G., Singh N. N., Stewart A. W., Field C. J. (1985). The aberrant behavior checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89, 485–491. - PubMed
-
- Benedetti F., Carlino E., Piedimonte A. (2016). Increasing uncertainty in CNS clinical trials: The role of placebo, nocebo, and Hawthorne effects. Lancet Neurology, 15, 736–747. - PubMed
-
- Bishop D. V. M. (2006). Children’s communication checklist-2 (CCC-2) (US Edition [Manual]). San Antonio, TX: Harcourt Assessments.
-
- Carrothers T. J., Periclou A., Khariton T., Ghahramani P. (2014). A population pharmacokinetic (PPK) model of memantine in pediatric patients with autistic spectrum disorder (ASD). J Pharmacokinet Pharmacodyn, 41(1 Suppl): S70.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

